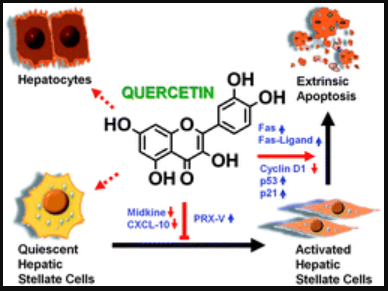
槲皮素 Quercetin Studies
蔬果汁中的槲皮素抗肝炎和肝纤维化的作用
inhibits NADPH oxidase derived oxidative stress via induction of heme oxygenase-1
The Flavonoid Quercetin Ameliorates Liver Inflammation and Fibrosis by Regulating Hepatic Macrophages Activation and Polarization in Mice
Xi Li1, Qianwen Jin2,3, Qunyan Yao2,3, Beili Xu2,3, Lixin Li3, Shuncai Zhang2,3* and Chuantao Tu2,3*
1Department of Geriatrics, Zhongshan Hospital, Fudan University, Shanghai, China
2Department of Gastroenterology and Hepatology, Zhongshan Hospital, Fudan University, Shanghai, China
3Shanghai Institute of Liver Diseases, Shanghai, China
At present, there are no effective antifibrotic drugs for patients with chronic liver disease; hence, the development of antifibrotic therapies is urgently needed. Here, we performed an experimental and translational study to investigate the potential and underlying mechanism of quercetin treatment in liver fibrosis, mainly focusing on the impact of quercetin on macrophages activation and polarization. BALB/c mice were induced liver fibrosis by carbon tetrachloride (CCl4) for 8 weeks and concomitantly treated with quercetin (50 mg/kg) or vehicle by daily gavage. Liver inflammation, fibrosis, and hepatic stellate cells (HSCs) activation were examined. Moreover, massive macrophages accumulation, M1 macrophages and their related markers, such as tumor necrosis factor (TNF)-α, interleukin (IL)-1β, IL-6, and monocyte chemotactic protein-1 (MCP-1) in livers were analyzed. In vitro, we used Raw 264.7 cells to examine the effect of quercetin on M1-polarized macrophages activation. Our results showed that quercetin dramatically ameliorated liver inflammation, fibrosis, and inhibited HSCs activation. These results were attributed to the reductive recruitment of macrophages (F4/80+ and CD68+) into the liver in quercetin-treated fibrotic mice confirmed by immunostaining and expression levels of marker molecules. Importantly, quercetin strongly inhibited M1 polarization and M1-related inflammatory cytokines in fibrotic livers when compared with vehicle-treated mice. In vitro, studies further revealed that quercetin efficiently inhibited macrophages activation and M1 polarization, as well as decreased the mRNA expression of M1 macrophage markers such as TNF-α, IL-1β, IL-6, and nitric oxide synthase 2. Mechanistically, the inhibition of M1 macrophages by quercetin was associated with the decreased levels of Notch1 expression on macrophages both in vivo and in vitro. Taken together, our data indicated that quercetin attenuated CCl4-induced liver inflammation and fibrosis in mice through inhibiting macrophages infiltration and modulating M1 macrophages polarization via targeting Notch1 pathway. Hence, quercetin holds promise as potential therapeutic agent for human fibrotic liver disease.Introduction
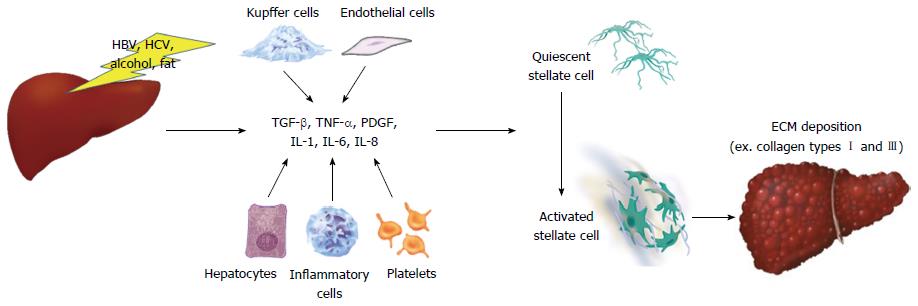
Liver fibrosis is a typical wound-healing process triggered by liver injury and inflammation resulting from a wide variety of etiologies, such as chronic virus infection (mainly hepatitis B and C viruses), alcoholic and nonalcoholic steatohepatitis (NASH), drugs, cholestasis, and autoimmune hepatitis (Friedman, 2008; Pellicoro et al., 2014; Tsuchida and Friedman, 2017). Nowadays, hepatic fibrosis is viewed as a dynamic process characterized by the massive excess deposition of extracellular matrix (ECM) in the liver (Friedman, 2008; Tsuchida and Friedman, 2017). It has been generally accepted that resident hepatic stellate cells (HSCs), which become activated and transdifferentiate into myofibroblast-like cells in response to chronic liver injury, are the major source of ECM during the process of liver fibrogenesis (Pellicoro et al., 2014; Seki and Schwabe, 2015; Tsuchida and Friedman, 2017). It has become evident that HSCs activation results from the inflammatory activity of liver immune cells, predominantly macrophages (Pellicoro et al., 2014; Seki and Schwabe, 2015; Li et al., 2017b). Of note, hepatic macrophages can directly mediate the behavior of HSCs and other myofibroblasts by producing a range of cytokines, chemokines, and other soluble mediates (Pellicoro et al., 2014). Additionally, activated myofibroblasts can amplify inflammatory responses by inducing the infiltration of macrophages and further secreting cytokines (Duffield et al., 2005; Pellicoro et al., 2014). Given the critical regulatory role of macrophages in HSCs activation and liver fibrosis, we believe that it provides therapeutic targets promising application in the future.The prevailing concept indicates that hepatic macrophages can arise either from proliferating resident macrophages, or from circulating bone marrow (BM)-derived monocytes, which are recruited to the injured liver (Duffield et al., 2005; Pellicoro et al., 2014; Wynn and Vannella, 2016). Macrophages are highly plastic cells that can be altered depending on the tissue microenvironment (Tacke and Zimmermann, 2014; Wynn and Vannella, 2016). Its polarization statue to M1 or M2 is often used to characterize macrophages; in which M1 macrophages exhibit an inflammatory phenotype while M2 macrophages are alternatively activated, including an anti-inflammatory phenotype (Beljaars et al., 2014; Sica et al., 2014; Tacke and Zimmermann, 2014; Tosello-Trampont et al., 2016). Moreover, increasing evidence suggests that M1 macrophages activation plays a critical role in liver inflammation and fibrosis (Beljaars et al., 2014; Sica et al., 2014; Tacke and Zimmermann, 2014). Additionally, inflammatory cytokines, including transforming growth factor-β1 (TGF-β1), tumor necrosis factor (TNF)-α, interleukin (IL)-1β, and IL-6, released from these cells trigger local inflammatory responses and perpetuate inflammation as well as HSCs activation (Sica et al., 2014; Tacke and Zimmermann, 2014; Tosello-Trampont et al., 2016). By contrast, emerging evidence suggested that alternative M2 macrophages attenuated hepatic steatosis and inflammation, and have a pivotal role in the resolution of fibrosis (Beljaars et al., 2014; Sica et al., 2014; Tosello-Trampont et al., 2016; Labonte et al., 2017).
Furthermore, macrophage polarization is regulated by several key molecular mechanisms, including epigenetic regulators, transcription factors, posttranscriptional regulators, and some signaling pathways (Sica and Mantovani, 2012; Sica et al., 2014; Wijesundera et al., 2014; Xu et al., 2015a). The switch in phenotypes determines their role in liver inflammation and fibrosis, thus controlling M1/M2 macrophage polarization provides potential targets for antifibrotic therapies (Sica et al., 2014; Xu et al., 2015a; Tacke, 2017). Moreover, it has been reported that the M1 macrophage phenotype was controlled by several molecular signaling or transcription factors, including Notch1 signaling, transducer and activator of transcription 1 (STAT1), and interferon-regulatory factor (IRF) 5 (Lawrence and Natoli, 2011; Sica et al., 2014; Xu et al., 2015a; Tacke, 2017), while IRF4 and STAT6 were shown to specifically regulate M2 macrophage polarization (Lawrence and Natoli, 2011). Thus, potential therapeutic approaches might aim to balance M1/M2 macrophages or to regulate macrophage polarization by targeting key macrophage transcription factors (Lawrence and Natoli, 2011; Sica and Mantovani, 2012; Wan et al., 2014; Labonte et al., 2017).
Quercetin (3,3′, 4′,5,7-pentahydroxyflavone; Figure 1A) is a well-known flavonoid widely found in many plants and fruits including apples, red grapes, citrus fruit, tomato, onions, and other leafy green vegetables, and a number of berries (Russo et al., 2012; Kim and Park, 2016). Quercetin is known to possess various biological and pharmacological activities including antioxidant, antiviral, anti-inflammatory, anti-proliferative, and antifibrotic effects (Marcolin et al., 2012; Russo et al., 2012; Li et al., 2016a). Indeed, the beneficial effects of quercetin on liver injury and fibrosis have been confirmed by several animal models (Hernandez-Ortega et al., 2012; Marcolin et al., 2012; Li et al., 2016b). Recently, we reported that quercetin inhibited liver inflammation and fibrosis in mice by modulating high mobile group box 1 (HMGB1) and toll-like receptor (TLR) 2/TLR4 signaling pathways (Li et al., 2016b).Results
Quercetin Inhibited Liver Inflammation and Fibrosis in CCl4-Treated Mice
Quercetin Inhibited Activation of HSCs in CCl4-Treated Mice
Quercetin Inhibited M1 Macrophage Polarization and Expression of Inflammatory Properties in Fibrotic Livers
Quercetin Attenuated M2 Macrophages Polarization and Expression of Immunosuppressive Genes in Fibrotic Livers
Quercetin Treatment Suppressed M1-Polarized Macrophages in Vitro
Quercetin Inhibited Hepatic Macrophages Activation and Suppressed M1-Polarization through Regulating the Expression Notch1 on Macrophages
Frontiers | The Flavonoid Quercetin Ameliorates Liver Inflammation and Fibrosis by Regulating Hepatic Macrophages Activation and Polarization in Mice | Pharmacology
https://www.frontiersin.org/articles/10.3389/fphar.2018.00072/full
Front. Cell Dev. Biol., 12 November 2018 | https://doi.org/10.3389/fcell.2018.00150
Role of Metabolism in Hepatic Stellate Cell Activation and Fibrogenesis
Wei Hou1,2 and Wing-Kin Syn2,3*
1Tianjin Second People’s Hospital and Tianjin Institute of Hepatology, Tianjin, China
2Division of Gastroenterology and Hepatology, Department of Medicine, Medical University of South Carolina, Charleston, SC, United States
3Section of Gastroenterology, Ralph H. Johnson Veterans Affairs Medical Center, Charleston, SC, United States
Activation of hepatic stellate cell (HSC) involves the transition from a quiescent to a proliferative, migratory, and fibrogenic phenotype (i.e., myofibroblast), which is characteristic of liver fibrogenesis. Multiple cellular and molecular signals which contribute to HSC activation have been identified. This review specially focuses on the metabolic changes which impact on HSC activation and fibrogenesis.
Introduction
Activation of hepatic stellate cells (HSCs) involves the transition from a quiescent to a proliferative, migratory and fibrogenic phenotype (i.e., myofibroblast) which is characteristic of liver fibrogenesis. To date, multiple cell-surface, cytoplasmic and nuclear molecular signals and pathways have been reported to modulate HSC activation, including cytokines (Syn et al., 2011, 2012); adipocytokines (Saxena and Anania, 2015; Coombes et al., 2016); Toll-like receptors (TLRs) (Chou et al., 2012; Seo et al., 2016); Interleukins (ILs) (Jiao et al., 2016); collagen receptors (Liu et al., 2017); nuclear receptors (Beaven et al., 2011; Ding et al., 2013; Li et al., 2014; Palumbo-Zerr et al., 2015; Duran et al., 2016); G protein-coupled receptors (GPCRs) (Li et al., 2015, 2016a; Le et al., 2018); autophagy (Thoen et al., 2011, 2012; Hernández-Gea and Friedman, 2012; Hernández-Gea et al., 2012); endoplasmic reticulum stress (Hernández-Gea et al., 2013; Koo et al., 2016); oxidative stress (Lan et al., 2015; Ou et al., 2018); epigenetics (Coll et al., 2015; Hyun et al., 2016; Kweon et al., 2016; Huang et al., 2018; Zheng et al., 2018); cell metabolism (Nwosu et al., 2016; Du et al., 2018; Franko et al., 2018; Zhang et al., 2018), etc. In addition, extracellular/paracrine signals from resident and inflammatory cells including hepatocytes (Zhan et al., 2006), macrophages (Pradere et al., 2013), natural killer cells (Glässner et al., 2012), natural killer T cells (Wehr et al., 2013), liver sinusoidal endothelial cells (LSECs) (Xie et al., 2012), platelets (Kurokawa et al., 2016), and B cells (Thapa et al., 2015) further promote HSC activation.
Frontiers | Role of Metabolism in Hepatic Stellate Cell Activation and Fibrogenesis | Cell and Developmental Biology
https://www.frontiersin.org/articles/10.3389/fcell.2018.00150/full
Quercetin attenuates the activation of hepatic stellate cells and liver fibrosis in mice through modulation of HMGB1-TLR2/4-NF-κB signaling pathways
Author links open overlay panelXiLiabQianwenJinaQunyanYaoaBeiliXuaZhengLicChuantaoTua
Show more
https://doi.org/10.1016/j.toxlet.2016.09.002Get rights and content
Highlights
•
Quercetin ameliorated CCl4-induced liver fibrosis in mice.
•
Quercetin inhibited hepatic stellate cells activation in vivo and in vitro.
•
Quercetin inhibited HMGB1-TLR2/TLR4 signaling pathways in fibrotic liver.
•
Quercetin suppressed nuclear translocation of NF-κB in fibrotic liver.Quercetin attenuates the activation of hepatic stellate cells and liver fibrosis in mice through modulation of HMGB1-TLR2/4-NF-κB signaling pathways - ScienceDirect
https://www.sciencedirect.com/science/article/abs/pii/S0378427416331319
Nutr Res Pract. 2016 Dec; 10(6): 623–628.
Published online 2016 Sep 9. doi: 10.4162/nrp.2016.10.6.623
PMCID: PMC5126412
PMID: 27909560
Induction of heme oxygenase-1 with dietary quercetin reduces obesity-induced hepatic inflammation through macrophage phenotype switching
Chu-Sook Kim,1 Hye-Seon Choi,2 Yeonsoo Joe,2 Hun Taeg Chung,2 and Rina Yucorresponding author1
Abstract
BACKGROUND/OBJECTIVES
Obesity-induced steatohepatitis accompanied by activated hepatic macrophages/Kupffer cells facilitates the progression of hepatic fibrinogenesis and exacerbates metabolic derangements such as insulin resistance. Heme oxyganase-1 (HO-1) modulates tissue macrophage phenotypes and thus is implicated in protection against inflammatory diseases. Here, we show that the flavonoid quercetin reduces obesity-induced hepatic inflammation by inducing HO-1, which promotes hepatic macrophage polarization in favor of the M2 phenotype.
MATERIALS/METHODS
Male C57BL/6 mice were fed a regular diet (RD), high-fat diet (HFD), or HFD supplemented with quercetin (HF+Que, 0.5g/kg diet) for nine weeks. Inflammatory cytokines and macrophage markers were measured by ELISA and RT-PCR, respectively. HO-1 protein was measured by Western blotting.
RESULTS
Quercetin supplementation decreased levels of inflammatory cytokines (TNFα, IL-6) and increased that of the anti-inflammatory cytokine (IL-10) in the livers of HFD-fed mice. This was accompanied by upregulation of M2 macrophage marker genes (Arg-1, Mrc1) and downregulation of M1 macrophage marker genes (TNFα, NOS2). In co-cultures of lipid-laden hepatocytes and macrophages, treatment with quercetin induced HO-1 in the macrophages, markedly suppressed expression of M1 macrophage marker genes, and reduced release of MCP-1. Moreover, these effects of quercetin were blunted by an HO-1 inhibitor and deficiency of nuclear factor E2-related factor 2 (Nrf2) in macrophages.
CONCLUSIONS
Quercetin reduces obesity-induced hepatic inflammation by promoting macrophage phenotype switching. The beneficial effect of quercetin is associated with Nrf2-mediated HO-1 induction. Quercetin may be a useful dietary factor for protecting against obesity-induced steatohepatitis.
Keywords: Obesity, quercetin, inflammation, macrophageInduction of heme oxygenase-1 with dietary quercetin reduces obesity-induced hepatic inflammation through macrophage phenotype switching
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5126412/
Arch Biochem Biophys. 2014 Dec 15;564:83-8. doi: 10.1016/j.abb.2014.09.005. Epub 2014 Sep 18.
Heme oxygenase-1 and anti-inflammatory M2 macrophages.
Naito Y1, Takagi T2, Higashimura Y3.
Author information
1
Molecular Gastroenterology and Hepatology, Graduate School of Medical Science, Kyoto Prefectural University of Medicine, Kyoto, Kyoto 602-8566, Japan. Electronic address: ynaito@koto.kpu-m.ac.jp.
2
Molecular Gastroenterology and Hepatology, Graduate School of Medical Science, Kyoto Prefectural University of Medicine, Kyoto, Kyoto 602-8566, Japan.
3
Molecular Gastroenterology and Hepatology, Graduate School of Medical Science, Kyoto Prefectural University of Medicine, Kyoto, Kyoto 602-8566, Japan; Department of Food Factor Science, Kyoto Prefectural University of Medicine, Kyoto, Kyoto 602-8566, Japan.
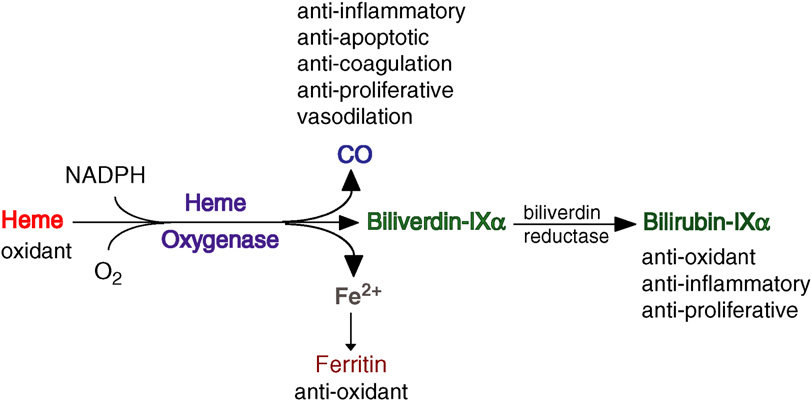
Abstract
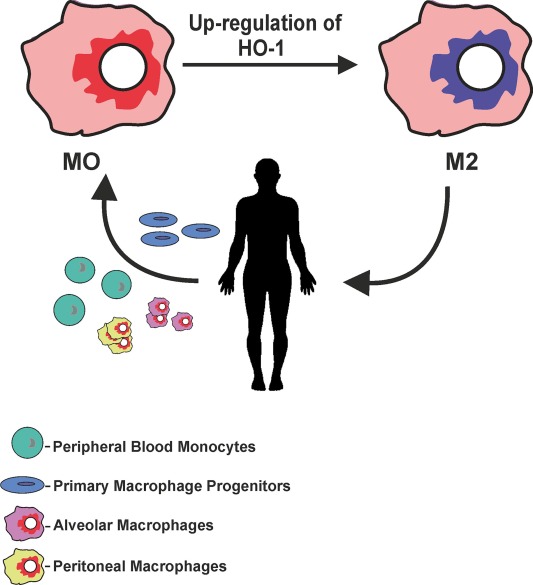
Heme oxygenase-1 (HO-1) catalyzes the first and rate-limiting enzymatic step of heme degradation and produces carbon monoxide, free iron, and biliverdin. HO-1, a stress-inducible protein, is induced by various oxidative and inflammatory signals. Consequently, HO-1 expression has been regarded as an adaptive cellular response against inflammatory response and oxidative injury. Although several transcriptional factors and signaling cascades are involved in HO-1 regulation, the two main pathways of Nrf2/Bach1 system and IL-10/HO-1 axis exist in monocyte/macrophage. Macrophages are broadly divisible into two groups: pro-inflammatory M1 macrophages and anti-inflammatory M2 macrophages. More recently, several novel macrophage subsets have been identified including Mhem, Mox, and M4 macrophages. Of these, M2 macrophages, Mhem, and Mox are HO-1 highly expressing macrophages. HO-1 has been recognized as having major immunomodulatory and anti-inflammatory properties, which have been demonstrated in HO-1 deficient mice and human cases of genetic HO-1 deficiency. However, the mechanism underlying the immunomodulatory actions of HO-1 remains poorly defined. This review specifically addresses macrophage polarization. The present current evidence indicates that HO-1 induction mediated by multiple pathways can drive the phenotypic shift to M2 macrophages and suggests that HO-1 induction in macrophages is a potential therapeutic approach to immunomodulation in widely diverse human diseases.
Copyright © 2014 Elsevier Inc. All rights reserved.
KEYWORDS:
Heme oxygenase; Inflammation; Macrophage; PolarizationHeme oxygenase-1 and anti-inflammatory M2 macrophages. - PubMed - NCBI
https://www.ncbi.nlm.nih.gov/pubmed/25241054
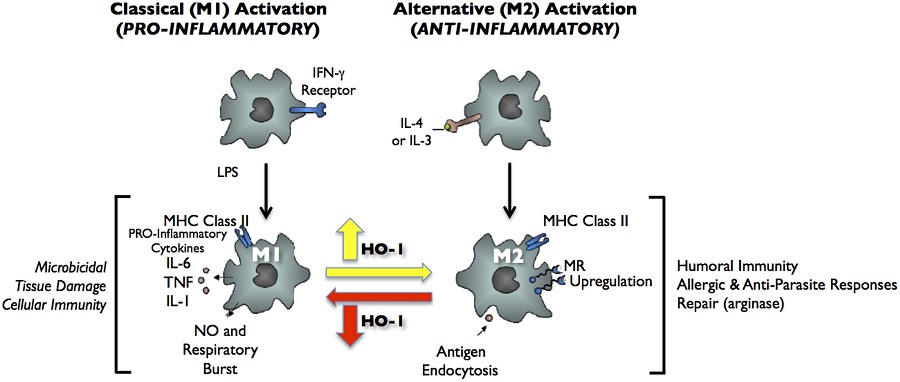
FIGURE 2. Classical activation (M1) and alternative activation (M2) of macrophages. Classical activation is mediated by the priming stimulus IFN-γ, followed by a microbial trigger (lipopolysaccharide, LPS). Alternative activation is mediated by IL- 4 or IL-13. The uptake of apoptotic cells or lysosomal storage of host molecules generates anti-inflammatory responses. Cytokines (IL-10, TGF-β, IFN-α/β) are potent modulators of activation.
Frontiers | Heme oxygenase-1 in pregnancy and cancer: similarities in cellular invasion, cytoprotection, angiogenesis, and immunomodulation | Pharmacology
https://www.frontiersin.org/articles/10.3389/fphar.2014.00295/full
Quercetin suppressed NADPH oxidase-derived oxidative stress via heme oxygenase-1 induction in macrophages
Author links open overlay panelMengjuanLuo1RongTian1ZiyiYangYi-YuanPengNaihaoLu
Key Laboratory of Functional Small Organic Molecule, Ministry of Education, Key Laboratory of Green Chemistry, Jiangxi Province and College of Chemistry and Chemical Engineering, Jiangxi Normal University, Nanchang, China
Highlights
•
Quercetin could induce HO-1 expression in macrophage cells.
•
Quercetin attenuated NADPH oxidase activity via a HO-1-dependent mechanism.
•
Quercetin suppressed LPS-induced inflammation in macrophages through HO-1 induction.
•
Suppression of NADPH oxidase activity was a novel protective mechanism for quercetin/HO-1 pathway.
Abstract
NADPH oxidase-derived superoxide (O2.-) generation and oxidative stress is usually considered as an important factor to the pathogenesis of inflammatory diseases. Quercetin, widely known for their anti-oxidant and anti-inflammatory properties in vitro and in vivo, is recently identified to induce expression of antioxidant enzyme heme oxygenase-1 (HO-1). Previous studies suggest that HO-1 induction and/or subsequent HO-1 end product generation in vitro and in vivo may suppress NADPH oxidase-derived oxidative stress. In this study, we tested whether quercetin might modulate NADPH oxidase activity in macrophages via induction of HO-1. In RAW264.7 macrophages, quercetin significantly attenuated NADPH oxidase-derived O2.- generation via a HO-1-dependent mechanism. Mechanistically, the protective effects of quercetin were (1) linked to increased expression of HO-1 in the presence or absence of lipopolysaccharide (LPS), (2) similar to that observed with the NADPH oxidase inhibitor apocynin, and (3) could be abolished by the specific small-interfering RNA against HO-1 expression or HO-1 activity inhibitor tin protoporphyrin. The induction of HO-1 by quercetin was associated with the nuclear accumulation of Nrf2 and downregulation of Keap1, a negative regulator of Nrf2. In addition, this flavonoid also inhibited the overproduction of nitric oxide and inflammatory cytokines in LPS-stimulated macrophages via simultaneous induction of HO-1 expression. In agreement with the observations in macrophages, pretreatment with quercetin significantly alleviated LPS-induced inflammation in mice which was concomitant with decreased NADPH oxidase activity and increased HO-1 expression. Our results suggested that quercein could modulate NADPH oxidase-derived O2.- production in macrophages at least partly through HO-1 induction. Suppression of NADPH oxidase-dependent oxidative stress may represent a novel mechanism underlying the anti-oxidant and anti-inflammatory properties of quercetin/HO-1 pathway.Quercetin suppressed NADPH oxidase-derived oxidative stress via heme oxygenase-1 induction in macrophages - ScienceDirect
https://www.sciencedirect.com/science/article/abs/pii/S0003986119303455
Quercetin Reduces Tumor Necrosis Factor Alpha-Induced Muscle Atrophy by Upregulation of Heme Oxygenase-1
Abstract
The inflammatory cytokine tumor necrosis factor α (TNFα), upregulated in the obese condition, promotes protein degradation and is implicated in obesity-related skeletal muscle atrophy and age-related sarcopenia. Quercetin, a flavonoid, elicits antioxidative and anti-inflammatory activities. In this study, we investigated the effect of quercetin on TNFα-induced skeletal muscle atrophy as well as its potential mechanism of action. In this study, we observed that quercetin suppressed expression of TNFα-induced atrophic factors such as MAFbx/atrogin-1 and MuRF1 in myotubes, and it enhanced heme oxygenase-1 (HO-1) protein level accompanied by increased nuclear translocation of nuclear factor erythroid 2-related factor 2 (Nrf2) in myotubes. The HO-1 inhibitor ZnPP suppressed the inhibitory actions of quercetin on TNFα-induced atrophic responses and degradation of IκB-α in myotubes. Moreover, quercetin supplementation to high-fat diet-fed obese mice inhibited obesity-induced atrophic responses in skeletal muscle, accompanied by upregulation of HO-1 and inactivation of nuclear factor-kappa B (NF-κB), and the quercetin actions were attenuated in Nrf2-deficient mice. These findings suggest that quercetin protects against TNFα-induced muscle atrophy under obese conditions through Nrf2-mediated HO-1 induction accompanied by inactivation of NF-κB. Quercetin may be used as a dietary supplement to protect against obesity-induced skeletal muscle atrophy.
https://www.liebertpub.com/doi/10.1089/jmf.2017.4108
These findings suggest that quercetin protects against TNFα-induced muscle atrophy under obese conditions through Nrf2-mediated HO-1 induction accompanied by inactivation of NF-κB. Quercetin may be used as a dietary supplement to protect against obesity-induced skeletal muscle atrophy.
Cited by: 5
Publish Year: 2018
Author: KimYeji, KimChu-Sook, JoeYeonsoo, ChungHun
World J Gastroenterol. Apr 21, 2017; 23(15): 2651-2659
Published online Apr 21, 2017. doi: 10.3748/wjg.v23.i15.2651
Quercetin Attenuates Inflammatory Responses in BV-2 ...
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4624710
Quercetin was 10 folds more potent than cyanidin in inhibition of lipopolysaccharide (LPS)-induced NO production as well as stimulation of Nrf2-induced heme-oxygenase-1 (HO-1) protein expression. In addition, quercetin demonstrated enhanced ability to stimulate HO-1 protein expression when cells were treated with LPS.
Cited by: 91
Publish Year: 2015
Author: Grace Y. Sun, Zihong Chen, Kimberly J. Jasmer, Dennis Y. Chuang, Zezong Gu, Mark Hannink, Agnes Simo...
Dietary quercetin ameliorates experimental colitis in ...
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5815442
Jan 02, 2018 · Dietary quercetin ameliorates experimental colitis in mouse by remodeling the function of colonic macrophages via a heme oxygenase-1-dependent pathway Songwen Ju , a, c, # Yan Ge , b, # Ping Li , c Xinxin Tian , d Haiyan Wang , b Xiaocui Zheng , b and Songguang Ju b
Hepatitis B virus infection and alcohol consumption
Ayako Iida-Ueno, Masaru Enomoto, Akihiro Tamori, Norifumi Kawada
Ayako Iida-Ueno, Masaru Enomoto, Akihiro Tamori, Norifumi Kawada, Department of Hepatology, Osaka City University Graduate School of Medicine, Osaka 545-8585, Japan
Hepatocellular carcinoma (HCC) is the most common type of primary liver cancer, and the second most common cause of cancer deaths worldwide. The top three causes of HCC are hepatitis B virus (HBV), hepatitis C virus (HCV), and alcoholic liver disease. Owing to recent advances in direct-acting antiviral agents, HCV can now be eradicated in almost all patients. HBV infection and alcoholic liver disease are expected, therefore, to become the leading causes of HCC in the future. However, the association between alcohol consumption and chronic hepatitis B in the progression of liver disease is less well understood than with chronic hepatitis C. The mechanisms underlying the complex interaction between HBV and alcohol are not fully understood, and enhanced viral replication, increased oxidative stress and a weakened immune response could each play an important role in the development of HCC. It remains controversial whether HBV and alcohol synergistically increase the incidence of HCC. Herein, we review the currently available literature regarding the interaction of HBV infection and alcohol consumption on disease progression.
Hepatitis B virus infection and alcohol consumption
https://www.wjgnet.com/1007-9327/full/v23/i15/2651.htm
Fibrogenic cell reversion underlies fibrosis regression in liver
Scott Laurence Friedman
PNAS June 12, 2012 109 (24) 9230-9231; https://doi.org/10.1073/pnas.1206645109
Related Articles
Myofibroblasts revert to an inactive phenotype during regression of liver fibrosis - Jun 12, 2012
Article Figures & SI Info & Metrics PDF
Significant progress in identifying cellular sources of ECM in experimental and human liver injury has led to a clearer understanding of fibrogenic cell dynamics during progressive fibrosis. Hepatic stellate cells, the resident perisinusoidal cell type that stores vitamin A, are the major source of ECM during diseases that injure hepatocytes (e.g., CCl4 in rodents and viral hepatitis in humans) through their activation into contractile myofibroblasts (1), and a similar transition to myofibroblasts from portal fibroblasts drives the fibrogenic response when biliary cells rather than hepatocytes are injured (2). The liver, with its unique regenerative capacity, has a remarkable ability to resorb scar when either hepatocellular or biliary injury is halted (3, 4). Reduced numbers of fibrogenic cells during fibrosis regression have been ascribed primarily to clearance of myofibroblasts through apoptosis (5), but the question has lingered of whether stellate cells can also revert to a quiescent state and persist as the liver’s normal architecture is restored. In the report by Kisseleva et al. (6) in PNAS, reversion is definitively established in two models of experimental liver injury using complementary genetic lineage tracing methods (Fig. 1).
Fibrogenic cell reversion underlies fibrosis regression in liver | PNAS
https://www.pnas.org/content/109/24/9230
Effects of luteolin and quercetin, inhibitors of tyrosine kinase, on cell growth and metastasis‐associated properties in A431 cells overexpressing epidermal growth factor receptor
Flavonoids display a wide range of pharmacological properties including anti‐inflammatory. Anti‐mutagenic, anti‐carcinogenic and anti‐cancer effects. Here, we evaluated the effects of eight flavonoids on the tumour cell proliferation, cellular protein phosphorylation, and matrix metalloproteinase (MMPs) secretion.
Of the flavonoids examined, luteolin (Lu) and quercetin (Qu) were the two most potent agents, and significantly inhibited A431 cell proliferation with IC50 values of 19 and 21 μM, respectively.
The epidermal growth factor (EGF) (10 nM) promoted growth of A431 cells (+25±4.6%) and mediated epidermal growth factor receptor (EGFR) tyrosine kinase activity and autophosphorylation of EGFR were inhibited by Lu and Qu. At concentration of 20 μM, both Lu and Qu markedly decreased the levels of phosphorylation of A431 cellular proteins, including EGFR.
A431 cells treated with Lu or Qu exhibited protuberant cytoplasmic blebs and progressive shrinkage morphology. Lu and Qu also time‐dependently induced the appearance of a ladder pattern of DNA fragmentation, and this effect was abolished by EGF treatment.
The addition of EGF only marginally diminished the inhibitory effect of luteolin and quercetin on the growth rate of A431 cells, treatment of cellular proteins with EGF and luteolin or quercetin greatly reduced protein phosphorylation, indicating Lu and Qu may act effectively to inhibit a wide range of protein kinases, including EGFR tyrosine kinase.
EGF increased the levels of matrix metalloproteinase‐2 (MMP‐2) and matrix metalloproteinase‐9 (MMP‐9), while Lu and Qu appeared to suppress the secretion of these two MMPs in A431 cells.Effects of luteolin and quercetin, inhibitors of tyrosine kinase, on cell growth and metastasis‐associated properties in A431 cells overexpressing epidermal growth factor receptor - Huang - 1999 - British Journal of Pharmacology - Wiley Online Library
https://bpspubs.onlinelibrary.wiley.com/doi/full/10.1038/sj.bjp.0702879