ˇˇ
ˇˇ
Macrophage polarization in obesity and type 2 diabetes: weighing down our understanding of macrophage function?
http://journal.frontiersin.org/article/10.3389/fimmu.2014.00470/full
ˇˇ
Nat Med. Author manuscript; available in PMC 2014 Oct 1.
Netrin-1 promotes adipose tissue macrophage accumulation and insulin resistance in obesity
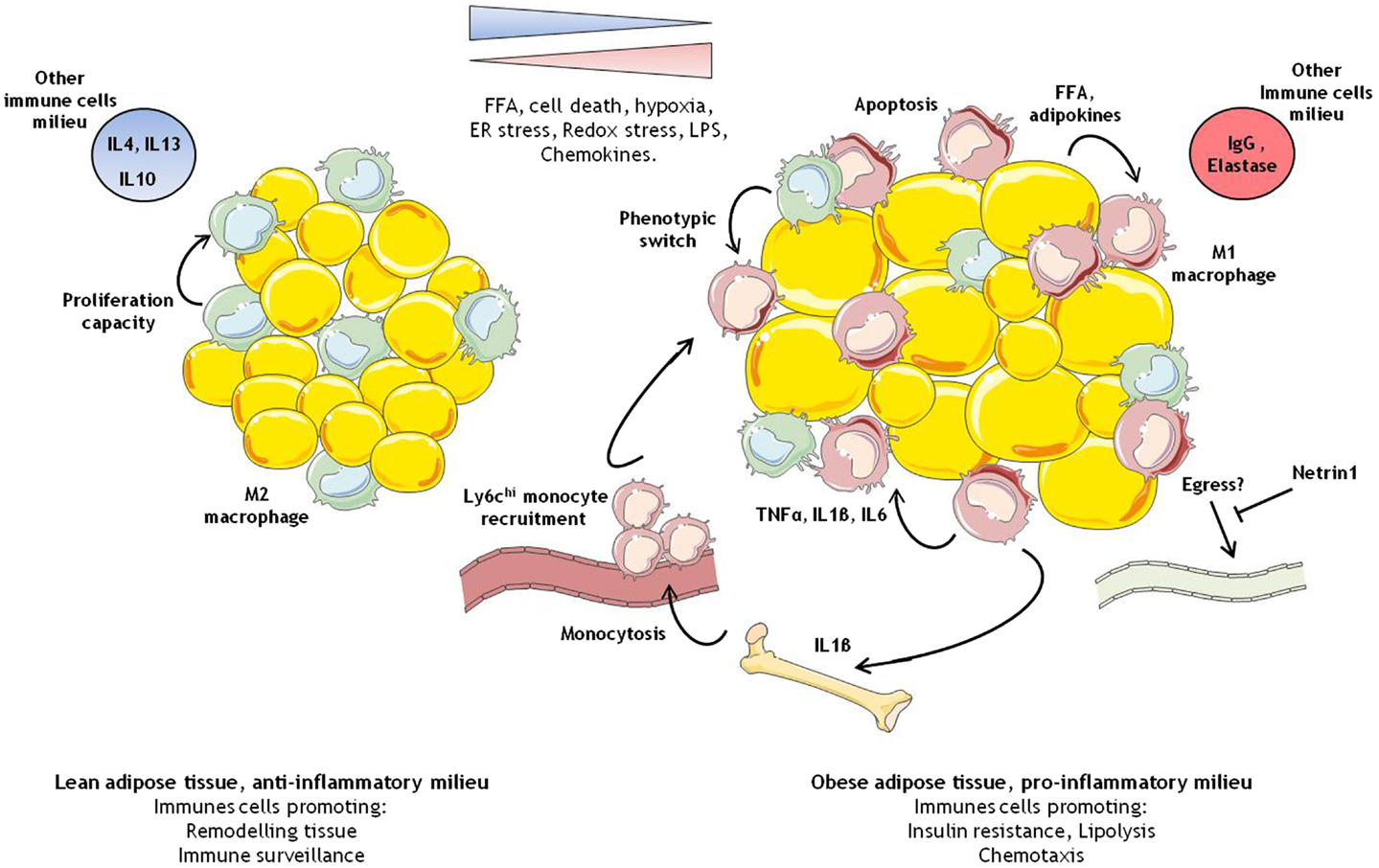
Abstract
During obesity, macrophage accumulation in adipose tissue propagates the chronic inflammation and insulin resistance associated with type 2 diabetes. The factors that regulate the accrual of macrophages in adipose are not well understood. Here we show that the neuroimmune guidance cue netrin-1 is highly expressed in obese, but not lean adipose tissue of humans and mice, where it directs the retention of macrophages. Expression of netrin-1 is induced in macrophages by the saturated fatty acid palmitate, and acts via its receptor Unc5b to block macrophage migration. In a mouse model of diet-induced obesity, we show that adipose tissue macrophages exhibit reduced migratory capacity, which can be restored by blocking netrin-1. Furthermore, hematopoietic deletion of Ntn1 facilitates adipose tissue macrophage emigration, reduces inflammation, and improves insulin sensitivity. Collectively, these findings identify netrin-1 as a macrophage retention signal in adipose tissue during obesity, which promotes chronic inflammation and insulin resistance.
Obesity and its co-morbidities, type 2 diabetes and cardiovascular disease, continue to increase and are major threats to global health. Studies in mice and humans have shown that expansion of adipose tissue mass is closely associated with the recruitment of cells of the myeloid and lymphoid lineage1¨C7, which gives rise to a state of chronic inflammation. The accumulation of adipose tissue macrophages (ATM) in obesity is striking, with ATMs comprising up to 40% of visceral white adipose tissue (VAT)5. These cells secrete pro-inflammatory molecules, including tumor necrosis factor alpha (TNF¦Á),8 interleukin-1 beta (IL-1¦Â)9,10, and CCL211, that contribute to the local and systemic inflammation that potentiate insulin resistance. The selective inhibition or genetic deficiency of factors that promote macrophage recruitment (eg. CCL2) or alter their inflammatory state (eg. IKKb) reduces adipose tissue inflammation and insulin resistance in obese mice11,12. In human studies, treatment of type 2 diabetics with the insulin-sensitizing thiazolidinediones showed a correlation between improved systemic insulin resistance and the reduction of ATMs and inflammatory factors13,14. These findings suggest that inflammation of the VAT compromises metabolic homeostasis. Resident tissue macrophages are a heterogeneous population, and their phenotype and function reflect their local metabolic and immune microenvironment. Macrophages that populate lean adipose tissue are thought to be similar to alternatively-activated or M2 macrophages15, which characteristically secrete anti-inflammatory cytokines (eg. IL-10) and promote tissue remodeling. With over-nutrition, increased numbers of classically activated or M1-like macrophages populate the VAT, where they secrete inflammatory factors that impair glucose homeostasis in this and other tissues16. Adipocyte-derived chemokines (eg. CCL217 and leukotriene B418) and obesity-associated increases in lipolysis19 are thought to provoke this influx of inflammatory monocyte-derived macrophages. However, studies have shown that the M1 and M2 macrophage phenotypes are not firmly entrenched, and interventions that alter key signaling molecules controlling alternative activation, such as the peroxisome proliferator activated receptor-¦Ă, can regulate the dynamic balance of ATMs and insulin sensitivity20.
ˇˇObesity is associated with macrophage accumulation in adipose tissue
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC296995/ˇˇ
J Clin Invest. 2003 Dec 15; 112(12): 1796¨C1808.
Obesity is associated with macrophage accumulation in adipose tissue
Abstract
Obesity alters adipose tissue metabolic and endocrine function and leads to an increased release of fatty acids, hormones, and proinflammatory molecules that contribute to obesity associated complications. To further characterize the changes that occur in adipose tissue with increasing adiposity, we profiled transcript expression in perigonadal adipose tissue from groups of mice in which adiposity varied due to sex, diet, and the obesity-related mutations agouti (Ay) and obese (Lepob). We found that the expression of 1,304 transcripts correlated significantly with body mass. Of the 100 most significantly correlated genes, 30% encoded proteins that are characteristic of macrophages and are positively correlated with body mass. Immunohistochemical analysis of perigonadal, perirenal, mesenteric, and subcutaneous adipose tissue revealed that the percentage of cells expressing the macrophage marker F4/80 (F4/80+) was significantly and positively correlated with both adipocyte size and body mass. Similar relationships were found in human subcutaneous adipose tissue stained for the macrophage antigen CD68. Bone marrow transplant studies and quantitation of macrophage number in adipose tissue from macrophage-deficient (Csf1op/op) mice suggest that these F4/80+ cells are CSF-1 dependent, bone marrow¨Cderived adipose tissue macrophages. Expression analysis of macrophage and nonmacrophage cell populations isolated from adipose tissue demonstrates that adipose tissue macrophages are responsible for almost all adipose tissue TNF-¦Á expression and significant amounts of iNOS and IL-6 expression. Adipose tissue macrophage numbers increase in obesity and participate in inflammatory pathways that are activated in adipose tissues of obese individuals.Obesity is associated with macrophage accumulation in adipose tissue
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC296995/ˇˇ