维生素C作为病毒性急性呼吸窘迫综合征的辅助治疗
CASE REPORT
1. dosage:
date 1:High-dose intravenous vitamin C (200 mg/kg per 24 h) was initiated on ECMO day 1 with the total daily vitamin C dosage divided equally into four doses and infused every 6 h.
date 2: reduced by half (100 mg/kg per 24 h) for one day following decannulation from ECMO then reduced by half again (50 mg/kg per 24 h) for an additional day. Post-extubation the patient required 4 L/min nasal oxygen for 48 h and then was discharged home on room air. She was discharged home on hospital day 12.
2. benefits:
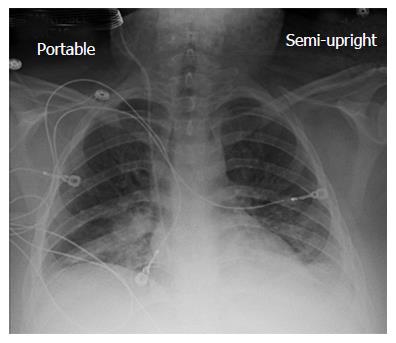
AP chest X-ray imaging on ECMO day 2 following institution of vitamin C infusion revealed significant improvement in bilateral lung opacities
lung gas exchange significantly improved following institution of vitamin C infusions.
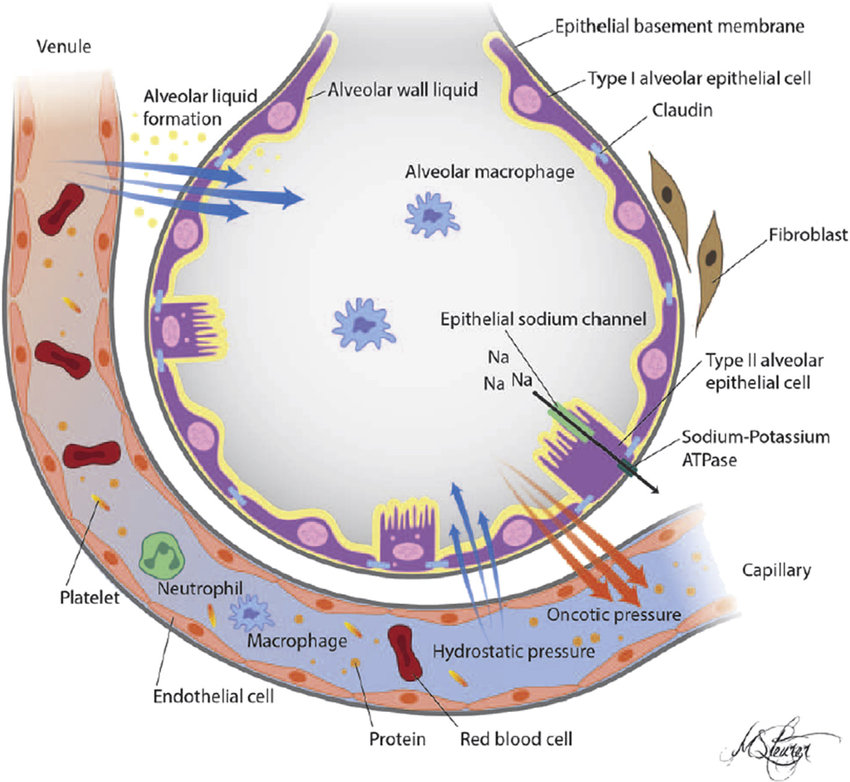
3. Na+ pumps serve as the engine powering vectorial Na+ transport across the AE and thus play an integral role in alveolar edema prevention and, if alveolar edema occurs, fluid clearance. Stretch up-regulate Na/K ATP pump~Implications~ benefit of breath holding
4. Widespread vascular endothelial injury is thought to be the major mechanism for multiorgan dysfunction and ARDS in sepsis,
World J Crit Care Med. 2017 Feb 4;6(1):85-90. doi: 10.5492/wjccm.v6.i1.85. eCollection 2017 Feb 4.
Intravenous vitamin C as adjunctive therapy for enterovirus/rhinovirus induced acute respiratory distress syndrome.
Fowler Iii AA1, Kim C1, Lepler L1, Malhotra R1, Debesa O1, Natarajan R1, Fisher BJ1, Syed A1, DeWilde C1, Priday A1, Kasirajan V1.
Author information
1
Alpha A Fowler III, Rajiv Malhotra, Orlando Debesa, Ramesh Natarajan, Bernard J Fisher, Aamer Syed, Christine DeWilde, Anna Priday, Division of Pulmonary Disease and Critical Care Medicine, Virginia Commonwealth University School of Medicine, Richmond, VA 23298, United States.
Abstract
We report a case of virus-induced acute respiratory distress syndrome (ARDS) treated with parenteral vitamin C in a patient testing positive for enterovirus/rhinovirus on viral screening. This report outlines the first use of high dose intravenous vitamin C as an interventional therapy for ARDS, resulting from enterovirus/rhinovirus respiratory infection. From very significant preclinical research performed at Virginia Commonwealth University with vitamin C and with the very positive results of a previously performed phase I safety trial infusing high dose vitamin C intravenously into patients with severe sepsis, we reasoned that infusing identical dosing to a patient with ARDS from viral infection would be therapeutic. We report here the case of a 20-year-old, previously healthy, female who contracted respiratory enterovirus/rhinovirus infection that led to acute lung injury and rapidly to ARDS. She contracted the infection in central Italy while on an 8-d spring break from college. During a return flight to the United States, she developed increasing dyspnea and hypoxemia that rapidly developed into acute lung injury that led to ARDS. When support with mechanical ventilation failed, extracorporeal membrane oxygenation (ECMO) was initiated. Twelve hours following ECMO initiation, high dose intravenous vitamin C was begun. The patient's recovery was rapid. ECMO and mechanical ventilation were discontinued by day-7 and the patient recovered with no long-term ARDS sequelae. Infusing high dose intravenous vitamin C into this patient with virus-induced ARDS was associated with rapid resolution of lung injury with no evidence of post-ARDS fibroproliferative sequelae. Intravenous vitamin C as a treatment for ARDS may open a new era of therapy for ARDS from many causes.静脉注射维生素C作为肠病毒/鼻病毒致急性呼吸窘迫综合征的辅助治疗。
Fowler Iii AA1, Kim C1, Lepler L1, Malhotra R1, Debesa O1, Natarajan R1, Fisher BJ1, Syed A1, DeWilde C1, Priday A1, Kasirajan V1。
作者信息 肺部疾病和重症监护医学部门,弗吉尼亚联邦大学医学院,里士满,VA 23298,美国。
摘要
我们报告一例由病毒引起的急性呼吸窘迫综合征(ARDS)患者接受肠外维生素C治疗。本报告概述了首次使用高剂量静脉注射维生素C作为介入治疗肠病毒/鼻病毒呼吸道感染所致ARDS。非常重要的临床前研究与维生素C和弗吉尼亚联邦大学执行之前执行的非常积极的结果第一阶段安全实验注入静脉注射高剂量维生素C为严重脓毒症患者,我们推断,注入相同剂量ARDS患者的病毒感染治疗。我们在此报告一位20岁以前健康的女性感染呼吸道肠病毒/鼻病毒,导致急性肺损伤并迅速发展为ARDS。她是在意大利中部的一个8-d春假期间感染的。在返回美国的航班上,她出现呼吸困难和低氧血症,并迅速发展为急性肺损伤,导致ARDS。当机械通气支持失败时,体外膜氧合(ECMO)开始启动。ECMO启动后12小时,开始高剂量静脉注射维生素C。病人恢复得很快。ECMO和机械通气在第7天停止,患者恢复,无长期ARDS后遗症。静脉注射高剂量的维生素C到病毒诱导的急性呼吸窘迫综合征患者体内与肺损伤的快速消退相关,但没有发现急性呼吸窘迫综合征后纤维增生性后遗症的证据。静脉注射维生素C作为ARDS的治疗手段,可能为ARDS的治疗开辟一个新的时代。Core tip: Enterovirus/rhinovirus has been reported to cause devastating acute lung injury. We report here the first use of high dose intravenous vitamin C to attenuate the acute respiratory distress syndrome that was caused by this viral infection. We have previously reported that vitamin C used in this interventional fashion is a potent anti-inflammatory agent.
Citation: Fowler III AA, Kim C, Lepler L, Malhotra R, Debesa O, Natarajan R, Fisher BJ, Syed A, DeWilde C, Priday A, Kasirajan V. Intravenous vitamin C as adjunctive therapy for enterovirus/rhinovirus induced acute respiratory distress syndrome. World J Crit Care Med 2017; 6(1): 85-90
URL: https://www.wjgnet.com/2220-3141/full/v6/i1/85.htm
DOI: https://dx.doi.org/10.5492/wjccm.v6.i1.85
KEYWORDS:
Acute lung injury; Acute respiratory distress syndrome; Enterovirus/rhinovirus; Extracorporeal membrane oxygenation; Intravenous vitamin C
PMID: 28224112 PMCID: PMC5295174 DOI: 10.5492/wjccm.v6.i1.85
Free PMC Article
INTRODUCTION
Viral diseases can produce the acute respiratory distress syndrome (ARDS)[1]. Pandemic viruses are the most common viruses that produce lung injury. Influenza viruses and coronaviruses (e.g., H5N1, H1N1 2009, severe acute respiratory syndrome coronavirus, and middle east respiratory syndrome coronavirus) are potentially lethal pathogens known to produce lung injury and death from ARDS[2-5]. At the tissue level, lung injury results from increased permeability of the alveolar-capillary membrane that leads to hypoxia, pulmonary edema, and intense cellular infiltration, particularly neutrophilic infiltration. The exact pathogenesis of virus-induced ARDS is slowly becoming understood. Unlike the “cytokine storm” occurring in bacterial sepsis that leads to up-regulation of pro-inflammatory cytokines [e.g., interleukin-1β (IL-1β), IL-8, IL-6] and generation of reactive nitrogen and oxygen species in the vascular space, viruses such as the influenza virus target alveolar epithelium, disabling sodium pump activity, damaging tight junctions, and inducing cell death in infected cells. Cytokines produced by virally infected alveolar epithelial cells activate adjacent lung capillary endothelial cells which then leads to neutrophil infiltration. Subsequent production of reactive oxygen and nitrogen species by infiltrating neutrophils further damages lung barrier function[1]. Apart from pandemic viruses other viruses, are increasingly reported to produce severe ARDS. While most of the approximately 100 strains of enterovirus primarily infect the gastrointestinal tract, enterovirus-D68 (EV-D68) has tropism for the respiratory tract. EV-D68 produces acute respiratory disease ranging from mild upper respiratory tract symptoms to severe pneumonia and lung injury as in the case we describe here. In an outpatient setting, EV-D68 disease has manifested most commonly among persons younger than 20 years and adults aged 50-59 years[6]. In August 2014, EV-D68 emerged as a cause of severe respiratory infections with hospitals in Illinois and Missouri reporting an increased incidence of rhinovirus and enterovirus infection[7]. In this report, 30 of 36 isolates from the nasopharyngeal secretions of patients with severe respiratory illness were identified as EV-D68. Following these reports, an unusually high number of patients with severe respiratory illness were admitted to these facilities, presumably with EV-D68 infection. Enterovirus-D68 leading to ARDS has been reported in China, Japan, and in the United States[8-11]. The report by Farrell et al[11], describes a previously healthy 26-year-old woman who developed severe ARDS following an enterovirus-D68 infection. Despite all critical care support measures, the patient required protracted mechanical ventilation for 32-d, necessitating tracheostomy and endoscopic gastrostomy tube placement. She was discharged alive 55 d following admission. Enterovirus and rhinovirus were recovered from the respiratory secretions of the patient we report here. Extracorporeal membrane oxygenation was rapidly required in our patient’s care following failure of conventional mechanical ventilation. The patient reported by Farrell et al[11] is the full extent of support required for patients with ARDS who ultimately develop a fibroproliferative course as described by Burnham et al[12]. Karhu et al[13] and Choi et al[14] recently reported finding rhinovirus as the etiology of severe community acquired pneumonia and respiratory failure in mechanically ventilated adults who had a proven viral etiology of respiratory failure.
We report here the first application of high dose intravenous vitamin C employed as an interventional drug treatment for virus-induced ARDS. Very few studies in critically ill patients with ARDS have reported the use of intravenous vitamin C. The use of vitamin C to treat lung injury is still investigational. Nathens et al[15] infused ascorbic acid at 1 g every 8 h combined with oral vitamin E for 28 d in 594 surgically critically ill patients and found a significantly lower incidence of acute lung injury and multiple organ failure. Tanaka et al[16] infused ascorbic acid continuously at 66 mg/kg per hour for the first 24 h in patients with greater than 50% surface area burns and showed significantly reduced burn capillary permeability. A single report (published as abstract only) of a clinical study of large intravenous doses of ascorbic acid, and other antioxidants (tocopherol, N-acetyl-cysteine, selenium), in patients with established ARDS showed reduction in mortality of 50%[17]. Clinical protocols currently in use for hospitalized septic patients fail to normalize ascorbic acid levels. Vitamin C dosages utilized in the treatment of the patient we describe in this case report arose from our previous human studies, infusing high dose intravenous vitamin C into critically ill patients with severe sepsis[18] and in our preclinical studies[19-21]. Our work thus far shows vitamin C to exert potent “pleotropic effects” when used as described in this report. We showed that septic patients receiving high dose intravenous vitamin C exhibit significant reduction in multiple organ injury and reduced inflammatory biomarker levels[18]. Our preclinical work in septic lung-injured animals shows that vitamin C down-regulates pro-inflammatory genes that are driven by transcription factor NF-κB. Furthermore, vitamin C significantly increases alveolar fluid clearance in septic lung-injured animals[21]. Finally, infused vitamin C’s capability to down-regulate liberated reactive oxygen and nitrogen species appears to be critical for attenuating lung injury[22].
CASE REPORT
A 20-year-old white female presented to urgent care with 24 h of increasing dyspnea after returning from a 7-d trip to Italy. While in Italy she was exposed to several members of the family with whom she was visiting who had symptoms of upper tract respiratory infection. One family member had recently traveled to Morocco. While in Italy, the patient had visited a buffalo farm and ate unpasteurized cheese. There were no other unusual exposures. She noted cough and yellow sputum for 3 d with intermittent fever and night sweats.
DISCUSSION
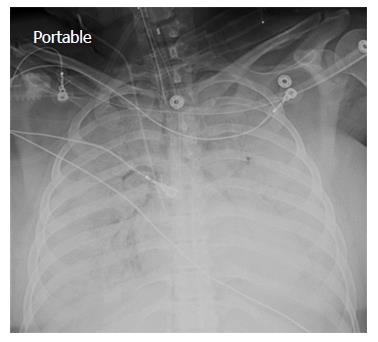
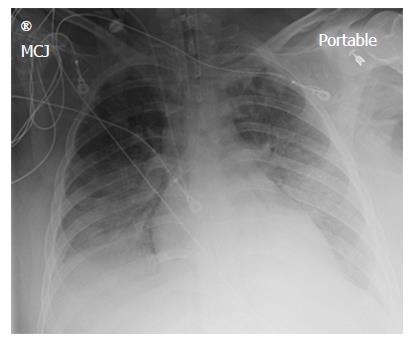
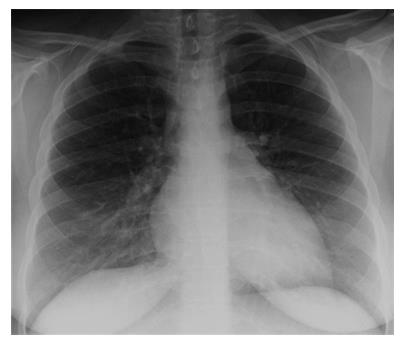
A chest X-ray revealed diffuse bilateral opacities (Figure 1). Arterial blood gas testing revealed severe hypoxemia while receiving 100% oxygen by non-rebreather mask. Antibiotics were initiated and she was admitted to intensive care unit (ICU) with a diagnosis of community acquired pneumonia. She denied GI symptoms, rash or arthralgia. She denied any history of thromboembolic disease, chest or leg pain or swelling. Her only medication was oral contraceptive for migraines associated with her menstrual cycle. Non-invasive positive pressure ventilation failed to support hypoxemic respiratory failure and intubation was required on hospital day 3. An echocardiogram revealed normal cardiac function. Respiratory cultures were negative, but a molecular detection viral respiratory panel was positive for enterovirus/rhinovirus (FilmArray, BioFire Diagnostics, LLC, Salt Lake City, Utah). Despite high PEEP and low tidal volume ventilation, hypoxemia (PaO2/FiO2 = 75) and hypercapnia remained severe. Chest imaging on hospital day 3 revealed dense bilateral opacities with central air bronchograms (Figure 2). Due to failure of conventional ventilatory strategies, veno-venous extracorporeal membrane oxygenation (ECMO) was initiated on hospital day 3. Low tidal volume assist-control, pressure-control ventilatory strategy was continued. Vancomycin, piperacillin-tazobactam and levofloxacin started at ICU admission were continued. High-dose intravenous vitamin C (200 mg/kg per 24 h) was initiated on ECMO day 1 with the total daily vitamin C dosage divided equally into four doses and infused every 6 h. AP chest X-ray imaging on ECMO day 2 following institution of vitamin C infusion revealed significant improvement in bilateral lung opacities (Figure 3). Given the patient’s hemodynamic instability and vasopressor requirements, the critical care physician staff and nursing staff were very careful to keep the patient’s intake and output fluid balance even, being careful not to volume load a patient who was suffering from permeability pulmonary edema. Bronchoscopy on ECMO day 3 was negative for bacterial or fungal respiratory pathogens. Histoplasma, Blastomyces, Aspergillus, and Legionella antigen studies were negative. Furosemide was used to achieve a daily negative fluid balance. Daily chest imaging with AP chest X-rays documented continued resolution of bilateral opacities. Importantly, lung gas exchange significantly improved following institution of vitamin C infusions. Chest imaging on ECMO day 6 revealed significant further reduction in lung opacities. ECMO decannulation and extubation from ventilation occurred on ECMO day 7 (Figure 4). Vitamin C dosing was continued while the patient remained on ECMO. Vitamin C dosing was reduced by half (100 mg/kg per 24 h) for one day following decannulation from ECMO then reduced by half again (50 mg/kg per 24 h) for an additional day. Post-extubation the patient required 4 L/min nasal oxygen for 48 h and then was discharged home on room air. She was discharged home on hospital day 12. Although we did not quantify the plasma ascorbic acid levels in the patient we report here, we have previously reported that critically ill patients with severe sepsis treated with the identical vitamin C infusion protocol achieved plasma ascorbic acid levels of 3.2 mmol, values which are 60 fold higher than normal plasma ascorbic acid levels[18].Figure 1 Patient’s anterior-posterior chest X-ray film prior to intubation.Figure 2 Patient’s anterior-posterior chest X-ray film on extracorporeal membrane oxygenation day 1.Figure 3 Patient’s anterior-posterior chest X-ray film on extracorporeal membrane oxygenation day 2.Figure 4 Patient’s anterior-posterior chest X-ray film on extracorporeal membrane oxygenation decannulation, extubation day 7.In conclusion, we report here the first use of vitamin C as an interventional drug to attenuate lung injury produced by viral infection. The patient described here was discharged home 12 d following hospitalization, requiring no oxygen therapy. Follow-up exam at 1 mo following the patient’s initial hospitalization revealed her to have completely recovered. Figure 5 displays her follow-up chest X-ray film. Importantly, it should be noted that this is a single case report. The role of Vitamin C in this patient’s recovery is not certain, and clearly additional investigation will be required before this can be recommended as a therapy for ARDS.
COMMENTS
Case characteristics
A 20-year-old female with no significant medical history presented with acute respiratory failure following a spring break in central Italy. While in Italy she was exposed to a sick contact who was a member of the family she was staying with.
Clinical diagnosis
The clinical diagnosis of severe acute respiratory distress syndrome (ARDS) in this case was established by the extent of respiratory failure present, the radiographic findings, and the need for extracorporeal membrane oxygenator support required. The patient’s exposure to the sick contact in Italy suggested the diagnosis of a viral etiology.
Differential diagnosis
ARDS, viral pneumonia, sepsis from unknown etiology.
Laboratory diagnosis
The diagnosis of the etiology of the patient’s respiratory failure was obtained by a panel that uses real-time polymerase chain reaction technology to identify respiratory viral pathogens. FilmArray Respiratory panel is manufactured by BioFire Diagnostics, LLC, Salt Lake City, Utah.
Imaging diagnosis
Standard Anterior-Posterior chest X-ray films confirmed the diagnosis of ARDS.
Pathological diagnosis
No lung tissue was obtained from the patient. The diagnosis of ARDS was established by the extent of respiratory failure and the imaging required during the patients hospital stay.
Treatment
In this case report, the authors describe the first use of high dosage intravenous vitamin C as adjunctive therapy for viral induced ARDS.
Related reports
At this point in time, there are no other case reports specifically referencing vitamin C as a treatment for ARDS. The authors have previously reported (ref. [18]) the use of high dose vitamin C as an adjunctive therapy for severe sepsis. Many patients in that trial likely could be considered to have had ARDS.
Experiences and lessons
For many years multiple investigators have conducted clinical treatment trials, searching for effective therapies to assist in the treatment for ARDS. In this case report, the authors may have shed new light on a treatment which may ultimately be effective. The successful outcome described in this case report would suggest that larger trials must be conducted with high dosage intravenous vitamin C.
Peer-review
This is an interesting report of use of high dose intravenous vitamin C in ARDS.Intravenous vitamin C as adjunctive therapy for enterovirus/rhinovirus induced acute respiratory distress syndrome
https://www.wjgnet.com/2220-3141/full/v6/i1/85.htmIntravenous vitamin C as adjunctive therapy for enterovirus/rhinovirus induced acute respiratory distress syndrome. - PubMed - NCBI
https://www.ncbi.nlm.nih.gov/pubmed/28224112
Na+-K+-ATPase activity in alveolar epithelial cells increases with cyclic stretch
Jacob L. Fisher , and Susan S. Margulies 01 OCT 2002https://doi.org/10.1152/ajplung.00030.2001
Abstract
Na+-K+-ATPase pumps (Na+ pumps) in the alveolar epithelium create a transepithelial Na+gradient crucial to keeping fluid from the pulmonary air space. We hypothesized that alveolar epithelial stretch stimulates Na+ pump trafficking to the basolateral membrane (BLM) and, thereby, increases overall Na+ pump activity. Alveolar type II cells were isolated from Sprague-Dawley rats and seeded onto elastic membranes coated with fibronectin or 5-day-conditioned extracellular matrix. After 2 days in culture, cells were uniformly stretched for 1 h in a custom-made device. Na+ pump activity was subsequently assessed by ouabain-inhibitable uptake of 86Rb+, a K+ tracer, and BLM Na+ pump abundance was measured. In support of our hypothesis, cells increased Na+pump activity in a “dose-dependent” manner when stretched to 12, 25, or 37% change in surface area (ΔSA), and cells stretched to 25% ΔSA more than doubled Na+ pump abundance in the BLM. Cells on 5-day matrix tolerated higher strain than cells on fibronectin before the onset of Na+ pump upregulation. Treatment with Gd3+, a stretch-activated channel blocker, amiloride, a Na+channel blocker, or both reduced but did not abolish stretch-induced effects. Sustained tonic stretch, unlike cyclic stretch, elicited no significant Na+ pump response.
RESULTS
Na+ pump activity increases with cyclic stretch in AE cells.
AE cells generally increase Na+ pump activity in response to mechanical strain, and they do so in a “dose-dependent” manner: the greater the applied strain, the greater the increase in Na+ pump activity.
the observation that stretch could still induce a smaller but significant increase in Na+ pump activity despite carefully administered Gd3+ or amiloride treatment indicates that this SAC-originating signaling pathway may not account for the entire response.
Tonic stretch has little effect on Na+ pump activity.
In contrast to cyclically stretched cells, AT2 cells stretched to 25% ΔSA and held at that strain for 1 h showed no significant stretch response (103 ± 17% relative to control values of 100 ± 12%; Fig. 4).
Cyclic stretch stimulates Na+ pump trafficking to the BLM.
To test the hypothesis that stretch increased Na+ pump activity by increasing the abundance of Na+ pumps in the plasma membrane, we compared Na+ pump content in BLMs of cells stretched cyclically for 1 h at 25% ΔSA with paired, unstretched controls. As shown in Fig. 6, Western blots of BLM Na+ pump α1-subunit from stretched and unstretched cells from three separate cell isolations show a marked increase (P < 0.01) in blot intensity with stretch with an average stretch-to-unstretched intensity ratio of 2.14. In contrast, whole cell homogenates of cells stretched cyclically for 1 h at 25% ΔSA showed no significant change in Na+ pump content. This indicates that stretch induces Na+ pump trafficking to the BLM from existing intracellular stores.
DISCUSSION
Na+ pumps serve as the engine powering vectorial Na+ transport across the AE and thus play an integral role in alveolar edema prevention and, if alveolar edema occurs, fluid clearance (43, 47, 64). Because acute alveolar edema is known to be present with the onset of VILI (22, 26, 36, 39, 54,71, 75), it is important to understand how mechanical strain of the AE, as experienced in mechanical ventilation, impacts the Na+ pumps that are responsible for preventing and resolving alveolar fluid accumulation.
The present study advances understanding of mechanically induced effects with evidence that Na+ pumps are trafficked to the cell surface and Na+ pump activity increases in a “dose-dependent” manner with cyclic strain of the AE membrane within physiological ranges. This indicates that alveolar edema occurs during VILI, despite increased pump activity, not because of it, at least in the short term. Such a result helps define the focus of edema resolution strategies by reducing concerns of dysfunctional Na+ pumps and emphasizing the overall imbalance between Na+ pumps working to clear fluids from the air space on one side and an overwhelming increase in epithelial permeability, which allows fluids to leak into the air space, on the other. Of course, this change of focus by no means discounts the importance of Na+pumps. Studies have clearly shown that stimulation or overexpression of Na+ pumps can greatly improve edema clearance in injured lungs (5, 6, 30, 31, 35, 40, 52, 61-63) above and beyond natural stretch-induced increases in Na+ pump activity, tipping the balance in favor of clearance, despite greatly increased alveolar permeability. Furthermore, recent studies indicate that Na+ pump function plays a critical role in tight junction formation and maintenance, underscoring Na+ pump importance not only on the clearance side of the balance but also on the permeability side (57).
With evidence of a stretch-activated Na+ pump upregulation, we focused on determining how the mechanical stretch stimulus is translated into an intracellular biochemical signal. SACs, a subclass of mechanogated channels, have been implicated as the origin of many biochemical cascades (33), including stretch-induced Na+ pump upregulation in a transformed murine lung epithelial cell line (MLE-12) (74). Using Gd3+, a lanthanide metal with potent SAC blocking potential, Waters et al. (74) abolished the stretch-induced Na+ pump response, implying an integral role of SACs in mediating Na+ pump upregulation in the MLE-12 cell line. In contrast, the present study in primary AE cells found that Gd3+ blocking significantly attenuated Na+ pump upregulation but did not abolish the effect completely. Hence, we conclude that SACs play an important role in stretch-induced Na+ pump upregulation but do not account for the total response in primary cells, which often respond to the stretch stimulus differently from immortal, transformed cell lines (68). Given that the SAC-modulated signal may not completely account for observed Na+ pump upregulation, it is possible that mechanotransduction through focal adhesions and the cytoskeleton (CSK) could contribute to the remainder of the observed increases in Na+ pump activity.
We also found that blocking ENaCs with amiloride or blocking with both Gd3+ and amiloride yielded results similar to treating with Gd3+ alone. This similarity between Gd3+ and amiloride and the apparent redundancy of both suggest that both toxins may be targeting the same channels, at least within the population of channels involved in stretch-induced Na+ pump stimulation. Caution is warranted, however, in concluding precisely which channels these are. Although amiloride is generally enlisted for blocking ENaCs and Gd3+ for SACs, both toxins block a broad, overlapping spectrum of channels, including voltage-gated and mechanogated ENaCs, L- and T-type Ca2+ channels, and numerous nonselective cation channels (33). Because the Na+ pump changes are associated with a stretch stimulus, we can conclude that the channels are stretch activated, but it is impossible to pinpoint an ion such as Na+ or to rule out ions such as Ca2+ as potential signalers.
Once stretch stimulates the signaling cascade, Na+ pump activity may be upregulated by increased activity of individual pumps or by an increase in the number of functional pumps in the BLM. On one hand, it is plausible that Na+ entering the cell through apical SACs provides increased substrate to Na+ pumps, thus increasing their velocity. On the other hand, previous studies of the AE using pharmaceutical stimulation (8, 63) or stretch (74) linked increased Na+ pump activity to increased Na+ pump content in the BLM. In this study, we observed increases in BLM Na+ pump content of slightly over twofold (∼214%) with cyclic stretch to 25% ΔSA; this corresponds nicely to observed increases in Na+ pump activity of the same order (∼236%) under the same conditions. However, we cannot completely rule out the possibility that individual Na+pump velocity could have increased slightly in response to increased substrate as well. Cell permeabilization, a technique that would create high intracellular Na+ substrate and thus ensure maximum Na+ pump velocity, was not used in this study, because group I cation ionophores such as nystatin and gramicidin are also permeable to 86Rb+. Thus permeabilization would decrease the ratio of 86Rb+ passing through Na+ pumps (signal) to 86Rb+ passing through ionophoric channels (noise), making it difficult to interpret data we obtain by this technique. Furthermore, observed increases in BLM Na+ pump content suggest that ionic influxes trigger a complex trafficking mechanism. This contraindicates permeabilization, because, in addition to ensuring maximum Na+ pump velocity, it could also artificially overstimulate Na+ pump trafficking. Thus we conclude from our data that overall Na+ pump activity clearly increases in proportion to an increase in Na+ pumps in the BLM, but we cannot conclude definitively whether increased Na+ pump velocity might also augment this response.
We also found that although stretch increased Na+ pump content in the BLM, overall levels of the protein remained constant. This implies that stretch stimulates Na+ pump recruitment from existing intracellular stores rather than de novo transcription and reflects what other investigators have observed by stimulating Na+ pump activity pharmacologically or mechanically (8, 63, 74). Similar to these studies in the literature, we observed that Na+ pump activity increases within 60 min, which corroborates the theory of fast trafficking from existing stores rather than slower production of new proteins (8, 63, 74).
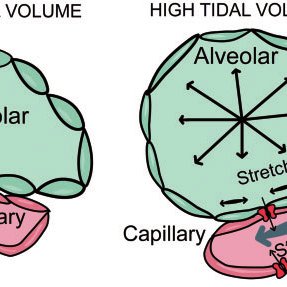
Differences in the Na+ pump response between cyclic and static stretch protocols may also provide insight into how Na+ pump activity increases with stretch and ultimately yield safer ventilation strategies. Our study shows that maintaining a static stretch of 25% ΔSA (80% total lung capacity) for 1 h has no significant impact on Na+ pump upregulation. Similarly, cyclic stretch from 12 to 25% ΔSA elicited a response similar to that caused by 12% ΔSA cyclic stretch and significantly less than that caused by 25% ΔSA cyclic stretch. This finding is consistent with viability results of Tschumperlin and Margulies (68), who observed a great reduction of AT2 cell mortality with tonic stretch relative to cyclic stretch to the same peak magnitude. Static deformations may provide an opportunity for the cell to reduce stress by remodeling the CSK via actin turnover (2) or the cell membrane via lipid trafficking (72). Cyclically stretched cells, in contrast, may not be held in the stretched position long enough to remodel their CSK or to traffic lipids and, thus, experience the full effect of stretch with each cycle. This implies that the application of a moderate tonic epithelial stretch, such as the baseline used in ventilation with positive end-expiratory pressure, has a small or, in the case of Na+ pump regulation, negligible effect on certain cell functions.
The present study also indicates that Na+ pump stretch response depends on the substratum on which the cells are tested. Although a positive correlation between Na+ pump activity and strain magnitude is evidenced on all substrata, cells grown on a 5-day ECM containing laminin, collagen, and fibronectin (27) tolerate a higher strain before the response appears than cells grown on fibronectin alone. Previous cell viability studies displayed a similar trend: cell mortality increased with strain, but cells grown on 5-day matrix tolerated higher strain than cells grown on fibronectin alone before similar mortality was observed (53). This apparent tolerance threshold may correspond to ECM-dependent modifications in the mechanical signal transduction path, such as different integrins involved, altered attachment protein density, or secondary CSK enhancements. An alternative explanation is that cells on 5-day matrix shifted phenotype more rapidly than cells on fibronectin and that the observed differences in Na+ pump response might reflect phenotype differences. However, because we detected no phenotype differences between cells on different substrata, it appears unlikely that phenotype accounts for substratum-dependent Na+ pump response.
The noted variation among cells on different substrata is indicative of the manifold challenges encountered in selecting a meaningful and tractable model. A variety of models have been used for testing the impact of stretch on Na+ pump regulation, but different models and methods have led to a variety of results. Sznajder et al. (67), looking at differences in ouabain-inhibitable ATP hydrolysis, reported an increase in Na+ pump activity in AT2 cells immediately after isolation from excised rat lungs that had been ventilated with high Vt for 25 min. However, in a similar study, Lecuona et al. (41, 42), using longer ventilation times (40 min), found a decrease in Na+ pump activity along with a corresponding decrease in edema clearance. On the other hand, Waters et al. (74) reported stretch-induced increases in Na+ pump activity in MLE-12 cells studied in vitro. The present study was designed to control for cell type, heterogeneity of applied deformation, and poststretch artifact and to focus on strain-magnitude effects. To that end, we used authentic primary cells, stretched the cells uniformly and equibiaxially at precise magnitudes, and assessed Na+ pump activity immediately after stretch with the cells still attached to the substratum.
Ultimately, the primary result of this study agrees with the studies of Waters et al. (74) with MLE-12 cells and with the studies of Sznajder et al. (67) with cells isolated from ventilated lungs but contrasts with the findings of Lecuona et al. (41, 42). Disparities possibly result from differences in the timing and treatment of cells after injury. Lecuona et al. stretched AT2 cells in situ by mechanical ventilation but then had to isolate AT2 cells from the lung before measuring uptake, whereas we were able to measure Na+ pump activity in an adherent, polarized epithelium immediately after stretch without otherwise disturbing the cells. Although the in vivo environment is clearly ideal for emulating VILI, it does not permit measurement of Na+pump activity immediately after stretch. Instead cells must undergo a lengthy and stressful isolation process, which removes them from the substratum completely, before Na+ pump activity can be evaluated. It has been shown that Na+ pumps are trafficked from intracellular stores and inserted into the cell membrane, and it is suggested that a reverse process of reabsorption from the membrane accounts for decreases in cellular Na+ pump activity (8); the stress of the isolation process and the disorientation of typically polar epithelial cells in suspension could possibly trigger such a reabsorption. Another possibility is that the two studies sample the same process at different time points. Lecuona et al. (42) note a decrease in Na+ pump activity but report no change in Na+ pump α1-subunit mRNA levels. The present study, evaluating the stretch effect immediately after stretch is ceased, notes increased pump activity but a reduction in mRNA, a possible prelude to the downstream scenario observed by Lecuona et al.
Although these differences in results primarily reflect disparity between the experimental models and methods used, they may also provide valuable insight into the underlying physiology. If Na+pumps are upregulated for the short term, but the intracellular mRNA is downregulated, the defense mechanism may be short lived. In addition, our data show that stretch reduces the intracellular store of ATP (12), the fuel required for the pumping process, so that stretch-induced Na+ pump upregulation may be a defense mechanism of only short-term efficacy.
In summary, we found that cyclic stretch of the AE stimulates ionic fluxes through SACs, which can be blocked by Gd3+ or amiloride. In response, the cell traffics Na+ pumps from intracellular stores to the cell membrane, where their augmented number serves to increase overall Na+ pump activity. Additionally, we report that stretch-induced upregulation of Na+ pump activity is subject to a number of factors, including substratum composition and stretch magnitude and mode. At the same time, we raise several questions that require further study, including why AT2 cells seem relatively insensitive to static stretch and what role integrins and CSK might play in signaling and Na+ pump trafficking. Finally, our study indicates that stretch-induced upregulation occurs but may not adequately counter the flux of fluids into the alveoli allowed by increased epithelial permeability or that the upregulation is possibly too short lived. Although it is not clear how this in vitro stretch-induced response translates into overall alveolar transport and why edema persists during VILI, our findings emphasize the importance of both sides of the balance, minimizing the initial disruption of epithelial integrity and sustaining Na+ pump upregulation, to reduce the incidence of alveolar edema.
Na+-K+-ATPase activity in alveolar epithelial cells increases with cyclic stretch | American Journal of Physiology-Lung Cellular and Molecular Physiology
https://journals.physiology.org/doi/full/10.1152/ajplung.00030.2001
Sepsis impairs alveolar epithelial function by downregulating Na-K-ATPase pump
Gidon Berger, Julia Guetta, Geula Klorin, Reem Badarneh, Eyal Braun, Vera Brod, … Show all Authors
01 JUL 2011https://doi.org/10.1152/ajplung.00010.2010
Abstract
Widespread vascular endothelial injury is the major mechanism for multiorgan dysfunction in sepsis. Following this process, the permeability of the alveolar capillaries is augmented with subsequent increase in water content and acute respiratory distress syndrome (ARDS). Nevertheless, the role of alveolar epithelium is less known. Therefore, we examined alveolar fluid clearance (AFC) using isolated perfused rat lung model in septic rats without ARDS. Sepsis was induced by ligating and puncturing the cecum with a 21-gauge needle. AFC was examined 24 and 48 h later. The expression of Na-K-ATPase proteins was examined in type II alveolar epithelial cells (ATII) and basolateral membrane (BLM). The rate of AFC in control rats was 0.51 ± 0.02 ml/h (means ± SE) and decreased to 0.3 ± 0.02 and 0.33 ± 0.03 ml/h in 24 and 48 h after sepsis induction, respectively (P < 0.0001). Amiloride, significantly decreased AFC in sepsis; conversely, isoproterenol reversed the inhibitory effect of sepsis. The alveolar-capillary barrier in septic rats was intact; therefore the finding of increased extravascular lung water in early sepsis could be attributed to accumulation of protein-poor fluid. The expression of epithelial sodium channel and Na-K-ATPase proteins in whole ATII cells was not different in both cecal ligation and puncture and control groups; however, the abundance of Na-K-ATPase proteins was significantly decreased in BLMs of ATII cells in sepsis. Early decrease in AFC in remote sepsis is probably related to endocytosis of the Na-K-ATPase proteins from the cell plasma membrane into intracellular pools, with resultant inhibition of active sodium transport in ATII cells.
sepsis and related systemic inflammatory reaction is a life-threatening disease that affects ∼750,000 patients a year in the United States; the frequency is increasing, given an aging population with increasing numbers of patients with comorbid conditions (29). The acute respiratory distress syndrome (ARDS) is a common, devastating clinical syndrome of acute lung injury (ALI) that affects both medical and surgical patients. Sepsis that is not caused by pneumonia is associated with the highest risk of progression to ALI or ARDS (12, 42). Widespread vascular endothelial injury is thought to be the major mechanism for multiorgan dysfunction and ARDS in sepsis, thereby augmenting the permeability of alveolar capillaries with subsequent influx of protein-rich edema fluid into the air spaces (17).
The resolution of both cardiogenic pulmonary edema and ARDS depends on the clearance of fluid from the alveolar space, a process that requires an intact, functional alveolar epithelium (22, 24). The primary driving force for alveolar fluid clearance (AFC) is the active transport of sodium from the alveolar space to the interstitium by alveolar epithelial type II cells (ATII cells) (2, 21, 23, 34, 36). Little is known about ATII function in sepsis. Therefore, we aimed to examine the effects of remote sepsis on AFC and active sodium transport.
Effects of Sepsis on Extravascular Lung Water and Permeability As depicted in Fig. 2A, the W/D lung weight ratio was increased in the 24-h sepsis group compared with control rats, 3.65 ± 0.38 and 2.27 ± 0.15, respectively (P = 0.017), indicating that the extravascular lung water was increased. At the same time, the permeability to large solutes as measured by the fraction of FITC-albumin that appeared in the alveolar space during the experimental protocol was not different in the control and 24-h sepsis groups, indicating that the alveolar-capillary barrier was intact and its permeability did not increase (Fig. 2B).
Fig. 2.A: wet-to-dry (W/D) lung weight ratios, a gravimetric estimate of total lung water, showed that in sepsis rat lungs there was a significant increase compared with control rat lungs. Values are means ± SE. *P < 0.05 compared with control rats. B: alveolar epithelial permeability to large particles (albumin) was not significantly changed in the study group compared with control rats.
In conclusion, AFC is decreased already in early stages of sepsis. Conceivably, this effect is due to endocytosis of the Na-K-ATPase proteins with resultant inhibition of active sodium transport and AFC, reflecting early dysfunction of the epithelial type II cell. The inhibitory effect of sepsis was restored following the administration of isoproterenol.
Sepsis impairs alveolar epithelial function by downregulating Na-K-ATPase pump | American Journal of Physiology-Lung Cellular and Molecular Physiology
https://journals.physiology.org/doi/full/10.1152/ajplung.00010.2010
Hospital-based Intravenous Vitamin C Treatment
for Coronavirus and Related Illnesses
by Andrew W. Saul and Atsuo Yanagisawa, MD, PhD
(OMNS February 2, 2020) No matter which hospital a coronavirus patient may seek help from, the question is, Will they be able to leave walking out the front door, or end up being wheeled out the basement backdoor? Prompt administration of intravenous vitamin C, in high doses, can make the difference.
Abundant clinical evidence confirms vitamin C's effectiveness when used in sufficient quantity. [1]
Physicians have demonstrated the powerful antiviral action of vitamin C for decades. [2]
Specific instructions for intravenous vitamin C
The Japanese College of Intravenous Therapy (JCIT) recommends intravenous vitamin C (IVC) 12.5/25g (12,500 - 25,000 mg) for acute viral infections (influenza, herpes zoster, common cold, rubella, mumps, etc.) and virus mimetic infections (idiopathic sudden hearing loss, Bell's palsy). In adults, IVC 12.5g is given for early stage illness with mild symptoms, and IVC 25g for moderate to severe symptoms. IVC is usually administered once or twice a day for 2-5 continuous days, along with or without general treatments for viral infections.
IVC 12.5g cocktail
Sterile water 125 mL
50% Vitamin C 25 mL (12. 5g)
0.5M Magnesium sulfate 10 mL
Add Vitamin B complex
Drip for 30-40 min
IVC 25g cocktail
Sterile water 250 mL
50% Vitamin C 50 mL (25g)
0.5M Magnesium sulfate 20 mL
Add Vitamin B complex
Drip for 40-60 min
Patients with acute viral infections show a depletion of vitamin C and increasing free radicals and cellular dysfunction. Such patients should be treated with vitamin C, oral or IV, for neutralizing free radicals throughout the body and inside cells, maintaining physiological functions, and enhancing natural healing. If patients progress to sepsis, vitamin C should be added intravenously as soon as possible along with conventional therapy for sepsis.
Toronto Star, 30 May 2003: "Fred Hui, MD believes that administering vitamin C intravenously is a treatment worth trying. And he'd like to see people admitted to hospital for the pneumonia-like virus treated with the vitamin intravenously while also receiving the usual drugs for SARS. 'I appeal to hospitals to try this for people who already have SARS,' says Hui. Members of the public would also do well to build up their levels of vitamin C, he says, adding that there is nothing to lose in trying it. 'This is one of the most harmless substances there is,' Hui states. 'There used to be concern about kidney stones, but that was theoretical. It was never borne out in an actual case.' Hui says he has found intravenous vitamin C effective in his medical practice with patients who have viral illnesses." [3]
Additional administration details are readily obtained from a free download of the complete Riordan Clinic Intravenous Vitamin C Protocol. [4] Although initially prepared for cancer patients, the protocol has found widespread application for many other diseases, particularly viral illnesses.
"Research and experience has shown that a therapeutic goal of reaching a peak-plasma concentration of ~20 mM (350- 400 mg/dL) is most efficacious. (No increased toxicity for posoxidant IVC plasma vitamin C levels up to 780 mg/dL has been observed.) . . . [T]he administering physician begins with a series of three consecutive IVC infusions at the 15, 25, and 50 gram dosages followed by post IVC plasma vitamin C levels in order to determine the oxidative burden for that patient so that subsequent IVCs can be optimally dosed."
Pages 16-18 of the Riordan protocol present IVC administration instructions.
http://www.doctoryourself.com/RiordanIVC.pdf or https://riordanclinic.org/wp-content/uploads/2015/11/RiordanIVCprotocol_en.pdf
There are four pages of supporting references.
"Given the rapid rate of success of intravenous vitamin C in viral diseases, I strongly believe it would be my first recommendation in the management of corona virus infections."
(Victor A. Marcial-Vega, MD)
Puerto Rico
"It is of great importance for all doctors to be informed about intravenous vitamin C. When a patient is already in hospital severely ill, this would be the best solution to help save her or his life."
(Karin Munsterhjelm, MD)
Finland
Winning the hospital game
When faced with hospitalization, the most powerful person in the most entire hospital system is the patient. However, in most cases, the system works on the assumption that the patient will not claim that power. If on your way in you signed the hospital's legal consent form, you can unsign it. You can revoke your permission. Just because somebody has permission to do one thing doesn't mean that they have the permission to do everything. There's no such thing as a situation that you cannot reverse. You can change your mind about your own personal healthcare. It concerns your very life. The rights of the patient override the rules of any institution.
If the patient doesn't know that, or if they're not conscious, or if they just don't have the moxie to do it, the next most powerful person is the spouse. The spouse has enormous influence and can do almost as much as the patient. If the patient is incapacitated, the spouse can, and must, do all the more. If there is no spouse present, the next most powerful people in the system are the children of the patient.
When you go to the hospital, bring along a big red pen, and cross out anything that you don't like in the hospital's permission form. And before you sign it, add anything you want. Write out "I want intravenous vitamin C, 25 grams per day, until I state otherwise." And should they say, "We're not going to admit you," you reply, "Please put it in writing that you refuse to admit me." What do you think their lawyers are going to do with that? They have to admit you. It's a game, and you can win it. But you can't win it if you don't know the rules. And basically, they don't tell you the rules.
This is deadly serious. Medical mistakes are now the third leading cause of death in the US. Yes, medical errors kill over 400,000 Americans every year. That's 1,100 each day, every day. [5]
There are mistakes of commission and mistakes of omission. Failure to provide intravenous vitamin C is, literally, a grave omission. Do not allow yourself or your loved ones to be deprived of a simple, easy to prepare and administer IV of vitamin C.
"If a family member of mine died due to coronavirus infection, after a doctor refused to use intravenous vitamin C, I would challenge his or her treatment in a court of law. I would win." (Kenneth Walker, MD, surgeon)
It can be done
Vitamin IVs can be arranged in virtually any hospital, anywhere in the world. Attorney and cardiologist Thomas E. Levy's very relevant presentation is free access. [6,7] http://www.doctoryourself.com/VC.NZ.Sept.2010.pdf and http://orthomolecular.org/resources/omns/v06n26.shtml.
Both the letter and the intent of new USA legislation now make this easier for you.
"The new federal Right to Try Act provides patients suffering from life-threatening diseases or conditions the right to use investigational drugs... It amends the Food, Drug, and Cosmetic Act to exempt investigational drugs provided to patients who have exhausted approved treatment options and are unable to participate in a clinical trial involving the drug. Advocates of right to try laws have sought to accelerate access to new drugs for terminally ill patients who are running out of options. Arguably, the law does not represent a radical change in this and several other states, however, because in 2016, California had already joined the majority of other states in adopting a law enabling physicians to help terminally ill patients pursue investigational therapies, without fear of Medical Board or state civil or criminal liability. . . The new Right to Try law should give physicians, as well as drug manufacturers, some added comfort about FDA enforcement in these cases." [8]
Therefore, in regards to intravenous vitamin C, do not accept stories that "the hospital can't" or "the doctor can't" or that "the state won't allow it." If you hear any of this malarkey, please send the Orthomolecular Medicine News Service the text of the policy or the law that says so. In the meantime, take the reins and get vitamin C in the veins.Intravenous Vitamin C Treatment, Coronavirus, hospital, high doses, antiviral, vitamin C, instructions for intravenous vitamin C, Intravenous Therapy, acute viral infections, influenza, herpes zoster, common cold, rubella, mumps, idiopathic sudden hearing loss, Bell's palsy, IVC, viral infections, Vitamin C, Magnesium sulfate, Vitamin B complex, depletion of vitamin C, therapy for sepsis, administering vitamin C intravenously, pneumonia-like virus, Riordan Clinic, Intravenous Vitamin C Protocol, cancer patients, articularly viral illnesses, peak-plasma concentration, IVC infusions, plasma vitamin C levels, Riordan protocol, IVC administration, viral diseases, hospitalization, Failure to provide intravenous vitamin C, coronavirus infection, Vitamin IVs, Right to Try Act
http://www.orthomolecular.org/resources/omns/v16n07.shtml