https://en.wikipedia.org/wiki/PDE5_drug_design
For every medication that is released into the world, there is often a ^natural remedy ̄ close behind it that claims to combat the same targeted issue. These remedies are derived from natural foraged plants or ingredients found in your local grocery store, and typically, only have anecdotal testimonials to back up their claims.
Top 6 Natural PDE-5 Inhibitors
https://www.nslij-genetics.org/natural-pde-5-inhibitors/
http://www.mikeblaber.org/oldwine/BCH4053/Lecture27/Lecture27.htm
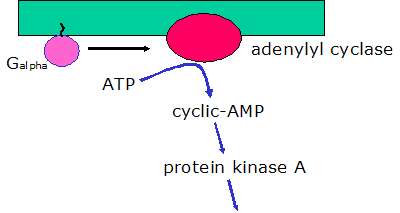
Cyclic adenosine monophosphate (cAMP, cyclic AMP, or 3',5'-cyclic adenosine monophosphate) is a second messenger important in many biological processes. cAMP is a derivative of adenosine triphosphate (ATP) and used for intracellular signal transduction in many different organisms, conveying the cAMP-dependent pathway.
https://alchetron.com/Cyclic-adenosine-monophosphate
J Immunology. 2005 Jan 15;
Cutting edge: macrophage inhibition by cyclic AMP (cAMP): differential roles of protein kinase A and exchange protein directly activated by cAMP-1
cAMP has largely inhibitory effects on components of macrophage activation, yet downstream mechanisms involved in these effects remain incompletely defined. Elevation of cAMP in alveolar macrophages (AMs) suppresses FcgammaR-mediated phagocytosis. We now report that protein kinase A (PKA) inhibitors (H-89, KT-5720, and myristoylated PKA inhibitory peptide 14-22) failed to prevent this suppression in rat AMs. We identified the expression of the alternative cAMP target, exchange protein directly activated by cAMP-1 (Epac-1), in human and rat AMs. Using cAMP analogs that are highly specific for PKA (N6-benzoyladenosine-3',5'-cAMP) or Epac-1 (8-(4-chlorophenylthio)-2'-O-methyladenosine-3',5'-cAMP), we found that activation of Epac-1, but not PKA, dose-dependently suppressed phagocytosis. By contrast, activation of PKA, but not Epac-1, suppressed AM production of leukotriene B(4) and TNF-alpha, whereas stimulation of either PKA or Epac-1 inhibited AM bactericidal activity and H(2)O(2) production. These experiments now identify Epac-1 in primary macrophages, and define differential roles of Epac-1 vs PKA in the inhibitory effects of cAMP.
The cAMP Pathway as Therapeutic Target in Autoimmune and Inflammatory Diseases
Department of Dermatology, University Medical Center Mainz, Johannes Gutenberg-University Mainz, Mainz, GermanyThe cAMP Pathway
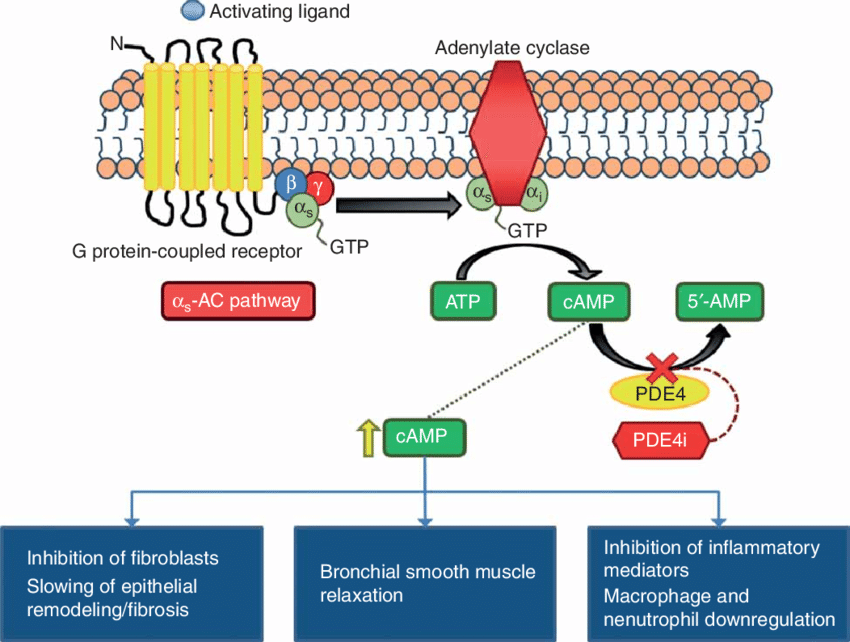
Cyclic adenosine monophosphate, identified in 1957 (2) as the first intracellular second messenger of extracellular ligand action, is now established as a universal regulator of metabolism and gene expression in all life forms (3). A family of enzymes called adenylate cyclases (AC) catalyzes cAMP formation from ATP.In vertebrates, AC comprise nine membrane-bound isoforms and one soluble isoform (4). AC vary in distribution and developmental expression and their regulation is complex and isozyme specific. In addition to AC expression and activity cAMP homeostasis is regulated by a superfamily of phosphodiesterases (PDE) that degrade intracellular cyclic nucleotides. PDE comprise more than 100 enzyme variants divided into 11 families (5) based on their structure, specificity for, and modulation by, cyclic nucleotides.
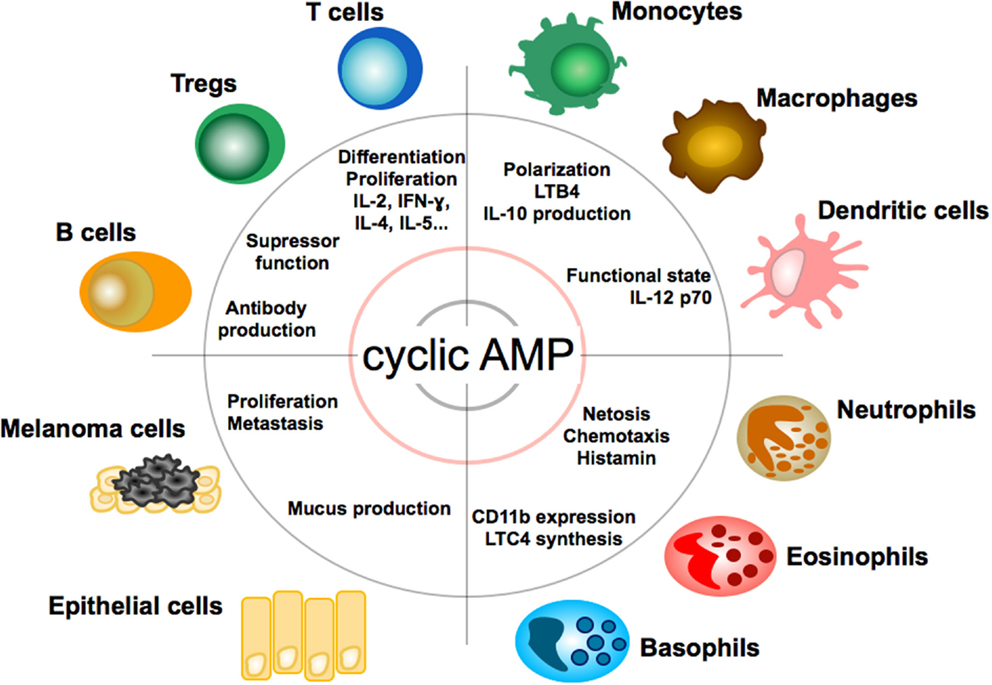
Figure 2. Effect of cAMP on immune, tumor, and epithelial cells. Impact and function of cyclic adenosin monophopshate (cAMP) on T and B lymphocytes, granulocytes, monocytes, macrophages, dendritic cells, epithelial cells, and melanoma cells. LTB4, leukotriene B4; LTC4, leukotriene C4.
http://journal.frontiersin.org/article/10.3389/fimmu.2016.00123/full
Phosphodiesterases (PDEs) are a superfamily of enzymes. This superfamily is further classified into 11 families, PDE1 - PDE11, on the basis of regulatory properties, amino acid sequences, substrate specificities, pharmacological properties and tissue distribution. Their function is to degrade intracellular second messengers such as cyclic adenine monophosphate (cAMP) and cyclic guanosine monophosphate (cGMP) which leads to several biological processes like effect on intracellular calcium level by the Ca2+ pathway.[1]
Phosphodiesterase 5 (PDE5) is widely expressed in several tissues in the body for example brain, lung, kidney, urinary bladder, smooth muscle and platelets.[1] It is possible to prevent cGMP hydrolysis by inhibiting PDE5 and therefore treat diseases associated with low cGMP levels, because of this, PDE5 is an ideal target for the development of inhibitors.[2] The therapeutic effects of PDE5 inhibition have been demonstrated in several cardiovascular conditions, chronic kidney disease and diabetes mellitus.[3]
The major PDE5 inhibitors (a subset of the phosphodiesterase inhibitors) are sildenafil, tadalafil, vardenafil, and avanafil, and although all share the same mechanism of action each has unique pharmacokinetic and pharmacodynamic properties which dictate their suitability in various conditions and their side effect profile.[3]
COPD: Current therapeutic interventions and future approaches
July 2005European Respiratory Journal 25(6):1084-106
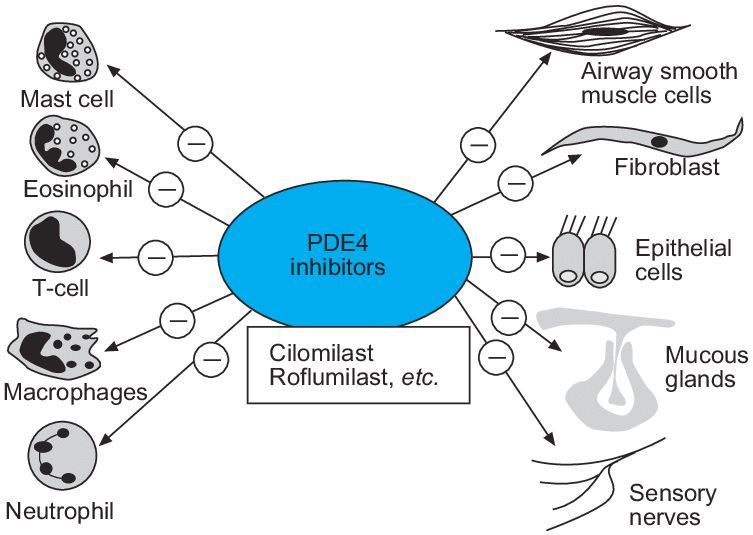
Although long-acting bronchodilators have been an important advance for the management of chronic obstructive pulmonary disease (COPD), these drugs do not deal with the underlying inflammatory process. No currently available treatments reduce the progression of COPD or suppress the inflammation in small airways and lung parenchyma. Several new treatments that target the inflammatory process are now in clinical development. Some therapies, such as chemokine antagonists, are directed against the influx of inflammatory cells into the airways and lung parenchyma that occurs in COPD, whereas others target inflammatory cytokines such as tumour necrosis factor-alpha. Broad spectrum anti-inflammatory drugs are now in phase III development for COPD, and include phosphodiesterase-4 inhibitors. Other drugs that inhibit cell signalling include inhibitors of p38 mitogen-activated protein kinase, nuclear factor-kappaB and phosphoinositide-3 kinase-gamma. More specific approaches are to give antioxidants, inhibitors of inducible nitric oxide synthase and leukotriene B(4) antagonists. Other treatments have the potential to combat mucus hypersecretion, and there is also a search for serine proteinase and matrix metalloproteinase inhibitors to prevent lung destruction and the development of emphysema. More research is needed to understand the cellular and molecular mechanisms of chronic obstructive pulmonary disease and to develop biomarkers and monitoring techniques to aid the development of new therapies.(PDF) COPD: Current therapeutic interventions and future approaches
https://www.researchgate.net/publication/7812091_COPD_Current_therapeutic_interventions_and_future_approaches
Natural Product Research 2019 May 29;
Natural phosphodiesterase 5 (PDE5) inhibitors: a computational approach
In 1998, sildenafil was marketed as the first FDA-approved oral drug for the treatment of erectile dysfunction (ED). During the last two decades, the commercialization of other synthetic phosphodiesterase 5 (PDE5) inhibitors has been paralleled by the rise of remedies based on natural molecules from different chemical classes (flavonoids, polyphenols and alkaloids in general). In this work, a set of in silico tools were applied to study a panel of 30 natural compounds claimed to be effective against ED in the scientific literature or in folk medicine. First, pharmacokinetic properties were analysed to exclude the compounds lacking in specific drug-like features. Estimated binding energy for PDE5 and selectivity towards other PDE isoforms were then considered to highlight some promising molecules. Finally, a detailed structural investigation of the interaction pattern with PDE in comparison with sildenafil was conducted for the best performing compound of the set.
Keywords: PDE5; docking; erectile dysfunction; flavonoids; sildenafil.Natural phosphodiesterase 5 (PDE5) inhibitors: a computational approach - PubMed
https://pubmed.ncbi.nlm.nih.gov/31140295/