﹛
﹛
Acidified Nitrite Cream in the Treatment of Tinea Pedis逋悢
﹛
﹛
Antimicrob Agents Chemother. 1996 Jun; 40(6): 1422每1425.
Antimicrobial effect of acidified nitrite on gut pathogens: importance of dietary nitrate in host defense
R S Dykhuizen, R Frazer, C Duncan, C C Smith, M Golden, N Benjamin, and C Leifert
Infection Unit, Aberdeen Royal Infirmary, United Kingdom.
ABSTRACT
Dietary intake of nitrate generates salivary nitrite, which is acidified in the stomach, leading to a number of reactive intermediates of nitrogen, among which are the potentially carcinogenic N-nitrosamines. Acidified nitrite, however, also has antimicrobial activity which coincides with the formation of nitric oxide. The present study examines the antimicrobial effect in vitro of acidified nitrite on Salmonella enteritidis, Salmonella typhimurium, Yersinia enterocolitica, Shigella sonnei, and Escherichia coli O157. First-order regression plots showed a linear inverse relationship of log-transformed proton and nitrite concentrations with MICs and MBCs after 30 min, 2 h, and 24 h of exposure (P < 0.001 for all antibacterial activities). Susceptibility to the acidified nitrate solutions ranked as follows: Y. enterocolitica > S. enteritidis > S. typhimurium = Shigella sonnei > E. coli O157 (P < 0.05). Addition of SCN-, but not that of CI-, increased the antibacterial activity (paired t testing, P < 0.001). Generation of salivary nitrite from dietary nitrate may provide significant protection against gut pathogens in humans.Antimicrobial effect of acidified nitrite on gut pathogens: importance of dietary nitrate in host defense.
﹛
Nitrogen oxides and nitrous acid are recognized in organic chemistry as noxious compounds and atmospheric pollutants and represent a significant population health risk (21). In humans, ingested nitrate (NO3 2) is absorbed from the gastrointestinal tract into the bloodstream and concentrated in the salivary glands by an active transport system shared with iodide and thiocyanate, increasing concentrations up to 10 times that in plasma (23, 25). Salivary nitrate is then rapidly reduced to nitrite (NO2 2) by nitrate reductase expressed by microorganisms in the mouth (11). N-Nitrosamines are formed from nitrite and secondary amines in the stomach (15, 20), and concerns about the endogenous formation of these potentially carcinogenic compounds has led to calls for restriction of nitrate and nitrite in food products and drinking water (24). We have recently suggested that the production of salivary
nitrite serves a useful purpose as a host defense mechanism against swallowed pathogens via the formation of bacteriocidal compounds in the stomach (1). It has been shown that expelled stomach air contains a high concentration of the antimicrobial
gas nitric oxide (NO步) which is enhanced by dietary nitrate intake (16). We proposed that the salivary generation of nitrite is accomplished by a symbiotic relationship involving nitratereducing bacteria on the tongue surface, which is designed to provide a host defense against microbial pathogens in the mouth and lower gut via chemical NO步 production (6). Patients with infective gastroenteritis have increased plasma nitrate levels compared with those in healthy controls (7), septicemic patients (19), and patients with inflammatory bowel disease (7a). During infective gastroenteritis, salivary generation of nitrite might be greatly enhanced, resulting in increased gastric NO步 production. To investigate the role of salivary nitrite in the bacteriocidal function of the stomach, we studied the effect of acidified nitrite on microorganisms involved in the etiology of infective gastroenteritis. Five microorganisms were tested by using acidification with hydrochloric acid and various concentrations of nitrite characteristic of concentrations found in saliva. We also studied the effects of other anions, including thiocyanate (which is also concentrated in saliva) and chloride (which is secreted into the gastric lumen) in combination with nitrite solutions acidified with sulfuric acid.﹛
MATERIALS AND METHODS
Production of standardized bacterial inocula. Patient isolates of Salmonella enteritidis, Salmonella typhimurium, Shigella sonnei, Yersinia enterocolitica, and Escherichia coli O157 were tested. All experiments used early-log-phase cultures. Flasks (125 ml) containing 75 ml of nutrient broth (Oxoid CM1) were inoculated and incubated on a shaker (New Brunswick Scientific Co., Edison, N.J.) for 18 h at 378C. The optical density was adjusted by dilution with fresh nutrient broth to produce a density of 2 3 107 CFU ml21
.
Determination of the bacteriostatic activity of acidified nitrite. The experiments were carried out on disposable, flat-bottom microwell plates (96 wells of 300 ml). Nitrite solutions to give a final concentrations of 0, 0.05, 0.1, 0.2, 0.5, 1, 2, and 10 mmol of nitrite per ml in the microwells and nutrient broth solutions acidified by hydrochloric acid to give final pHs of 5.4, 4.8, 4.2, 3.7, 3.0, and 2.1 were prepared. The microwells were filled with nitrite solution (60 ml), bacterial suspension (60 ml), and acidified nutrient broth (120 ml). The plates were sealed and incubated for 24 h at 378C on the shaker. The inhibitory effect of acidified nitrite on bacterial growth was determined by measurement of the optical density (570 nm) of the wells using a microwell plate reader (MRX Microplate Reader; Dynatech Products Ltd., Guernsey, Channel Islands, Great Britain). To determine the effect of Cl2 and SCN2 on the antimicrobial activity of acidified nitrite, the experiment was repeated with S. enteritidis using acidification by sulfuric acid (H2SO4) with 10 mM NaCl, Na2SO4, or NaSCN in the microwells. All experiments were carried out in triplicate. Determination of the bacteriocidal activity of acidified nitrite. After 30 min, 2 h, and 24 h of exposure to acidified nitrite, 20 ml of the bacterial suspensions was transferred to 180 ml of a recovery medium (nutrient broth; pH 5 7.0). From this first transfer, a further 20 ml was transferred to recovery medium to accomplish neutralization of acid, dilution of nitrite concentration, and reduction of the original inoculum size to a final number of 10,000 microorganisms. Recovery media were incubated on the shaker for 24 h at 378C before assessment of microbial growth with the microwell plate reader. Interpretation of results and statistical analysis. The MIC of NO2 2 at thedifferent pH settings was defined as the lowest NO2 2 concentration at which no growth of microorganisms had taken place after 24 h. The MBC was defined as the lowest NO2 2 concentration at which no growth was detected after transfer into recovery media. MICs and MBCs of nitrite (in micromoles per milliliter) were log transformed for statistical analysis. Differences in susceptibility of mi-croorganisms to nitrite acidified with HCl were assessed by analysis of variance and paired t testing for means of the MIC, MBC after 30 min exposure time
(MBC0.5h), MBC2h, and MBC24h at the six pH values applied in the experiments. The same method was used to assess the differences in susceptibility of S. enteritidis to nitrite acidified with sulfuric acid with 10 mM Na2SO4, NaCl, or NaSCN present in the solution. Paired t testing was also applied to compare the mean concentrations of nitrite required to accomplish bacteriostasis, and bacterial killing after 30 min, 2 h, and 24 h of exposure. The slopes of the regression curves of acidified nitrite for the different microorganisms and antibacterial activities were assessed by regression analysis.﹛
﹛
RESULTS
The means of the nitrite concentrations showing antimicrobial activity at pHs 2.1, 3, 3.7, 4.2, 4.8, and 5.4 are summarized in Table 1. Y. enterocolitica, S. enteritidis, and S. typhimurium were all killed at pH 2.1 after 30 min of exposure. Shigella sonnei and E. coli O157 survived, unless nitrite was present in the solution (0.20 and 1 mmol/ml, respectively [Table 1]). A linear relationship between log[NO2
2] and pH was present for MIC, MBC0.5h, MBC2h, and MBC24h between pHs 2.1 and 4.8 (Fig. 1). The cumulative R for the regression lines was significant for all antimicrobial activities (P , 0.001). Regression analysis showed a significantly steeper slope for the MIC
regression line compared with those for MBC0.5h (P 5 0.007) and MBC2h (P 5 0.032). At a pH of $4.8, the nitrite required to achieve bacterial killing after short exposure times (MBC0.5h and MBC2h) was frequently .10 mmol/ml and the linear relationship between
log[NO2] and pH was lost. There was a significant difference between MBC0.5h, MBC2h, MBC24h, and MIC at all pH settings (paired t testing for means, P , 0.001). Analysis of variance showed significant differences in susceptibility to acidified nitrite between individual organisms (P , 0.001). At pH 2.1, E. coli O157 was significantly less susceptible compared with all other microorganisms (paired t testing, P , 0.001), and throughout the pH range, analysis of regression showed the slope of its regression line to be significantly lower (P , 0.001). The susceptibilities of S. typhimurium and Shigella sonnei were not significantly different. S. enteritidis was more susceptible than S. typhimurium (paired t testing P , 0.001), and Y. enterocolitica was most susceptible of all microorganisms tested (P 5 0.047 compared with S. enteritidis and P , 0.001 compared with all other microorganisms). Adding 10 mmol of NaSCN per liter to the microwell resulted in a significant reduction of the amount of acidified nitrite required to accomplish activity (Table 2) (paired t testing, P , 0.001). Addition of NaCl or Na2SO4 resulted inidentical antibacterial activities.﹛
﹛
DISCUSION
Acidification of nitrite will lead to generation of reactive intermediates of nitrogen that have cytotoxic properties (Fig.2). At a given pH value, the quantity of NO步 generated in vitro is dependent on the nitrite concentration (6). In the solutions used during this experiment, a nitrite concentration of 0.01 mmol/ml at pH 3 generated a peak concentration of nitric oxide of 1 ppm in the headspace and a nitrite concentration of 1.2 mmol/ml generated 10 ppm. Within this range of nitrite concentrations at pH 3, a bacteriocidal effect within 2 h of exposure was accomplished for all microrganisms (Table 1), suggesting that gastric contents generating 1- to 10-ppm NO步 would have a bacteriocidal effect on these gut pathogens within the transit time of a food bolus through the stomach. In vivo measurements of NO步 production in the human stomach have shown values between 1 and 180 ppm, depending on dietary nitrate intake (5, 16). Nitric oxide inhibits respiratory chain enzymes through inactivation of iron-sulfur complexes (9) and disrupts DNA replication by inhibiting ribonucleotide reductase (17). Its toxicity has been demonstrated for a rapidly expanding list of microorganisms (3) as well as for tumor cells (18). However, experiments with NO步 donor compounds have shown little antibac terial activity of NO步 itself (4), and its toxic effects are mor likely to be accomplished via the formation of peroxynitrite in the presence of superoxide (29), the oxygen-dependent generation of the nitrogen dioxide radical when nitric oxide concentrations are high (2), and/or the formation of still-uncharacterized nitrogen species (28). It seems most likely that the antibacterial activity of acidified nitrite is due to an additive contribution of reactive intermediates of nitrogen (12).
﹛
﹛
Susceptibilities to the acidified nitrite solutions ranked as follows: Y. enterocolitica . S. enteritidis . S. typhimurium 5 Shigella sonnei. E. coli O157 was different in its response to acidified nitrite compared with the other four microorganisms; in the absence of nitrite it was significantly more resistant to acid, but addition of nitrite seemed to abolish this difference (Table 1). In conclusion, addition of nitrite to acidic solutions achieves killing of gut pathogens where acid alone allows growth to continue. Physiological concentrations of nitrite accomplish killing after exposure times that are comparable with the transfer time of a food bolus through the stomach. Addition of thiocyanate, which is also concentrated in saliva, but not of chloride increased antibacterial activity (Table 2). Generation of salivary nitrite increases greatly after nitrate ingestion, suggesting that ingestion of foods rich in nitrate protects against infective gastroenteritis. The high plasma nitrate levels observed in patients that are suffering from infective gastroenteritis may protect against the fecal-oral route of reinfection via increased generation of salivary nitrite. This mechanism may limit the impact of outbreaks of gastroenteritis, which would be relevant in humans but also would be of particular importance in other mammalian species and animal husbandry.
Health-conscious individuals and government authorities have advocated restriction of dietary nitrates for the last 20 years after ingestion of amines and nitrates had been associated with gastric cancer in animal models. Although the harmful and potentially carcinogenic activity of N-nitrosamines cannot be dismissed, epidemiological evidence for this association has been lacking (8, 13). We submit that the mechanism of chemical host defense which seems to take place in symbiosis with nitrate-reducing bacteria on the surface of our tongues may be of fundamental importance. Rather than a potential carcinogen, we postulate that nitrate may be a useful nutrient, particularly when accompanied by ascorbic acid (26), as is the case with vegetables. A conclusive demonstration of the antimicrobial effect of acidified nitrite in vivo would require a major reinterpretation of the role of dietary nitrate in human
health and animal husbandry.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC163343/401422.pdf
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC163343/pdf/401422.pdf﹛
BMC Res Notes. 2011; 4: 458.
An observational prospective study of topical acidified nitrite for killing methicillin-resistant Staphylococcus aureus (MRSA) in contaminated wounds
Anthony D Ormerod,corresponding author1 Amjad AJ Shah,2 Hong Li,3 Nigel B Benjamin,4 Gail P Ferguson,1 and Carlo Leifert5
Author information Article notes Copyright and License information Disclaimer
1Division of Applied Medicine, University of Aberdeen, Polwarth Building, Foresterhill, Aberdeen, AB24 2ZD, UK
2Royal Lancaster Infirmary, Ashton Road, Lancaster, LA1 4RP, UK
3Centre For Ecology & Hydrology, Lancaster Environment Centre, Bailrigg, Lancaster, LA1 4AP, UK
4Acute Medical Unit, Level 9, Derriford Hospital, Plymouth, PL6 8DH, UK
5Newcastle University, Nafferton Farm, Stocksfield, Northumberland, NE43 7XD, UK
corresponding authorCorresponding author.
Anthony D Ormerod: ku.ca.ndba@doremro.d.a; Amjad AJ Shah: ku.shn.thbm.ilr@hahS.deyS; Hong Li: ku.ca.hec@gnohil; Nigel B Benjamin: ku.shn.tsews.tnhp@nimajneB.legiN; Gail P Ferguson: ku.ca.ndba@nosugref.g; Carlo Leifert: ku.ca.lcn@trefiel.c
Abstract
Background
Endogenous nitric oxide (NO) kills bacteria and other organisms as part of the innate immune response. When nitrite is exposed to low pH, NO is generated and has been used as an NO delivery system to treat skin infections. We demonstrated eradication of MRSA carriage from wounds using a topical formulation of citric acid (4.5%) and sodium nitrite (3%) creams co-applied for 5 days to 15 wounds in an observational prospective pilot study of 8 patients.
Findings
Following treatment with topical citric acid and sodium nitrite, 9 of 15 wounds (60%) and 3 of 8 patients (37%) were cleared of infection. MRSA isolates from these patients were all sensitive to acidified nitrite in vitro compared to methicillin-sensitive S. aureus and a reference strain of MRSA.
Conclusions
Nitric oxide and acidified nitrite offer a novel therapy for control of MRSA in wounds. Wounds that were not cleared of infection may have been re-contaminated or the bioavailability of acidified nitrite impaired by local factors in the tissue.
Background
The widespread clinical use of antibiotics over the last 50 years has led to the emergence of resistant strains [1]. Methicillin-resistant Staphylococcus aureus (MRSA) was first noted in Great Britain in the early 60 s [2]. MRSA is a major cause of infections in humans worldwide, in both the community and the hospital [3]. Following targeted action, the incidence of MRSA bacteraemia has been falling since 2006 in the UK [3]. However, it still remains a considerable problem throughout Europe [4]. Surgical wounds are frequently colonised or significantly infected with MRSA [5]. MRSA is a major cause of surgical site infection which can delay hospital discharge [6]. In another study 23% of diabetic foot ulcers were infected [7]. MRSA infections are also a frequent cause of abscesses [8] and novel or better bactericidal agents that can be applied to wounds for decolonisation or prevention are urgently needed [9]
Acidified nitrite was devised as a novel means of liberating the bactericidal gas nitric oxide (NO) on the skin as a topical antibiotic therapy [10-12]. Briefly nitrite and hydrogen ions form nitrous acid (1) which is converted to dinitrogen trioxide (2), which dissociates into nitric oxide and nitrous oxide (3).
NO2- + H+ ⇄ HNO2 (1)
2HNO2 ⇄ H2O + N2O3 (2)
N2O3 ⇄ NO + NO2 (3)
We found that Trichophyton mentagrophytes, T. rubrum, Candida albicans, Streptococcus pyogenes, S. aureus and Propionibacterium acnes are all sensitive to acidified nitrite [12], with S. aureus being particularly sensitive. In the clinic, the concept of combining topical treatment with a nitrite containing cream and an acidic cream as a means of topical NO therapy has been proven [11,10] and used to treat Mycobacterium ulcerans causing Buruli ulcer [13] by co-application of creams containing 6% sodium nitrite and 9% citric acid.
As topical NO combining 3.0% (w/v) sodium nitrite and 4.5% (w/v) citric acid also facilitates experimental wound healing [14] it is an excellent candidate for decontaminating infected wounds. We hypothesised that acidified nitrite would be a useful agent in eliminating MRSA infection from the skin and aimed to demonstrate its ability to inhibit and kill MRSA in vitro and in a clinical plot study.
Methods
Subjects
We recruited hospitalised patients with a positive MRSA wound culture. Pregnant and lactating females and those with carriage of MRSA cultured from nose, axilla, groins, throat or sputum were excluded from the study to avoid recontamination of the wound. Patients taking systemic antibiotics or demonstrating additional pathogens in the wound swabs were also excluded.
Initial swabs of the nose, throat, axillae, perineum and any unhealed wounds were taken to assess eligibility for the study. These were all repeated in a routine fashion by the nursing staff in accordance with well defined infection control protocols at baseline, day five of treatment and two and four days after stopping topical acidified nitrite therapy to assess recurrence of infection. Those who developed nasal carriage during the study were treated with nasal applications of mupirocin.
Intervention
Treatment was applied to the infected wound twice daily for 5 days. Trained nursing staff co-applied equal amounts of 4.5% citric acid in aqueous cream and 3% sodium nitrite in aqueous cream mixed directly on the infected wound and surrounding skin which was then covered with a light gauze dressing. Once daily dressings were changed using aseptic technique, wounds were irrigated with sterile saline and the cream and a sterile dressing reapplied.
Safety
Acidified nitrite has been used safely in previous studies [11,13,15,16]. Citric acid and sodium nitrite were chosen as having optimum stability and safety for use in humans, citric acid being a naturally occurring agent in the diet and an approved ingredient in cosmetics and skin care products. Sodium nitrite also occurs naturally in the diet in vegetables and meat and is a permitted food preservative, but systemic doses of 4.5 g are sufficient to kill a human through methaemoglobinaemia. There have also been concerns about systemic formation of N-nitrosothiols which may have carcinogenic potential in the gut [17]. The concentrations of sodium nitrite and citric acid used represent a 2:1 molar ratio of sodium nitrite to citric acid to ensure sufficient acid is available to react with the nitrite to prevent residual nitrite being systemically absorbed leading to methaemoglobinaemia. Citric acid and sodium nitrite have been used safely for topical application to the skin up to 9 and 13.5% twice daily for up to 4 months in unpublished studies (Pro-Strakan data on file) and on ulcerated skin has been used safely in humans up to 9% and 6% respectively in a published study [18], where positive effects on wound healing were observed. Two year carcinogenicity studies have been performed on 330 mice without significant oncogenesis. In pilot studies and Phase 1&II studies in man over 700 subjects have received skin treatment with concentrations of acidified nitrite of up to 9% sodium nitrite and 13.5% citric acid for up to 3-4 months with no treatment related serious adverse events and only mild skin irritation and staining occurring as side effects (Pro-Strakan data on file).
MRSA Screening
All clinical samples were processed by standard culture methods on horse blood agar and MacConkey agar. Suspect colonies were confirmed as S.aureus by the slide coagulase test (Staph latex kit; Prolex Neston UK) and by the antibiogram using Clinical and Laboratory Standards Institute. Surveillance samples were processed by enrichment and culture. Swabs were placed in nutrient broth (Oxoid, Baskingstoke, UK) supplemented with 10% salt and incubated in ambient air for 18-24 hours. The broth was then subcultured on to Columbia agar (Oxoid) supplemented with 4 mg/L methicillin and incubated for a further 18-24 h in ambient air. All suspect colonies from surveillance swabs were confirmed as MRSA by the slide coagulase test and antibiogram [19].
Typing of isolates
The first MRSA isolate from each subject was used for in vitro testing and was phenotyped by the Scottish MRSA reference library using PCR and pulsed field gel electrophoresis. The phenotypes of the seven MRSA strains were again confirmed using coagulase, DNAase and methicillin susceptibility tests. The coagulase test was performed according the instruction of ProlexTM Staph Latex Kit (Pro-Lab Diagnostics, UK). For the DNASE test, overnight cultures were spotted onto DNASE agar (Oxoid, UK) and incubated for 37∼C for 24 hours. A clear zone around the colony indicated MRSA positive strains. The methicillin susceptibility test was conducted whereby an ME E-test strip (Cambridge Diagnostic Services Ltd. UK) was placed on the surface of the agar freshly swabbed with the test strain. After incubation of the agar at 30∼C for 24 hours, the methicillin-induced inhibition zone was recorded.
In vitro sensitivity
All MRSA bacterial strains and an MSSA reference strain were grown in either nutrient broth (Sigma-Aldrich) or on nutrient agar (1.5% w/v) at 37∼C. To determine the antimicrobial activity of acidified nitrite, using HCl as the acid, the assay was performed as described previously [12,20,21]. In brief, the pH of the nutrient broth was adjusted with HCl as this was previously standardized as an assay to create a pH gradient ranging from 1.7 to 7.0 across a micro-titre plate. To achieve a gradient of acidified nitrite, a gradient of potassium nitrite ranging from 0 to 10000 米M was then set up along the length of the micro-titre plate. For all experiments, 8 ℅ 107 cells ml-1 of a stationary phase culture of each strain was used as an inoculum. The micro-titre plate was then incubated at 37*C, 90 rpm and after the designated time, 20 米l aliquots were removed, serially diluted and colony forming units determined by assessing growth on nutrient agar after an overnight incubation at 37∼C. The inhibitory effect of acidified nitrite on bacterial growth was determined by measurement of optical density (570 nm) (MRX Microplate Reader, Dynatech) after 24 hours. The minimum inhibition concentration (MIC) for each strain were determined as the average values from 5 replicates. The MIC of acidified nitrite was defined as the lowest nitrite concentration whereby no growth of the strains had taken place at a certain pH after 24 hours.
The protocol was approved by the local Grampian Combined Ethical Committee and Infection Control Committees.
Results
Patients meeting the entry criteria with MRSA colonisation confined to a wound, with negative swabs elsewhere were rare. Recruiting from an acute hospital with over 1000 beds over 18 months, this limited the study to 8 patients, 6 of whom had more than one colonised wound giving a total of 15 infected wounds. Their clinical details are summarised in table table1.1. These patients were all treated at the wound sites only with 4.5% citric acid co-administered with 3% sodium nitrite for 5 consecutive days. Swabs were regularly performed from wounds and from other skin sites (see methods). Application of acidified nitrite at these concentrations was well tolerated by all subjects and there was no irritation of the skin noted or increase in pain from the wounds.
Table 1
Clinical details and outcomes of wound cultures from subjects treated with acidified nitrite where more than one wound was treated this is shown a several rows.
Age Sex Immuno-supressed Strain tested in vitro Wounds Necrosis Cultures from wound day: Other sites becoming positive
0 5 7 9
84 M No 3 Gangrene toe Yes + + + + No
90 F No 4 Amputation No + - - - No
83 F Prednisolone azathioprine 5 Vasculitic
Leg ulcers Yes + + + + No
+ + - -
85 M No 6 Orthopaedic pin sites No + - - - No
+ - - -
74 F No 7 Pressure sores No + + + + Yes Axilla
(day 5)
+ + + +
74 M No 8 Orthopaedic pin sites No + - - - No
+ + + +
79 F Prednisolone Not tested Infected blisters No + - - - No
+ - - -
+ - - -
68 F Renal failure Not tested Calciphylaxis ulcers on legs Yes + - - + Yes groins
(day 7)
+ + + +
Open in a separate window
Following treatment, three patients were completely cleared of their wound infection without recurrence after a further 4 days. Three showed a partial response (clearance of one wound) and two failed to respond. Of the 15 infected wounds 9 (60%) were cleared of MRSA colonisation. Of wounds that responded only 2/9(22%) were necrotic and non-responders were necrotic in 4/6(66%) this difference was not statistically significant (Fisher's exact test).
Although precautions were taken to cover the wounds and use sterile technique when the dressings were changed, re-contamination could occur when dressings were changed or when wetted in the shower. Non-response might also be explained by such recontamination from untreated areas of the patient or close environment. This is supported by the observation that 3 patients developed positive swabs from other previously negative untreated body sites during the study. Four of 7 (57%) of wounds still colonised or recolonised at day 9 occurred in patients with other sites becoming positive while 8 of 8 (100%) of wounds that remained clear occurred in patients not becoming positive at other sites (p = 0.077 Fisher's exact test).
The first isolate from each patient was used to confirm the phenotype of the MRSA isolate and for In vitro testing (Table (Table2).2). These showed similar or greater in vitro sensitivity to MSSA as measured by MIC at identical pH and nitrite concentrations (Figure (Figure1).1). As with the MIC analysis, we found that the minimum bactericidal concentration (MBC) of NO2- was reduced as the pH of the medium was lowered. In addition, we found that the average MBC to acidified nitrite for the seven MRSA strains was slightly lower than for the MSSA strain, indicating that MRSA strains do not have increased resistance to acidified nitrite. This was particularly evident at pH 4.5, since no killing of the MSSA strain was observed after 2 hours using the maximum concentration of sodium nitrite used in our assay (Figure (Figure2);2); in contrast, the MRSA strains were still killed under these conditions.
Table 2
Typing of isolates.
Strain Coagulase test DNASE test Methicillin susceptibility test* Reference lab
MRSA
Phenotype MRSA Genotype
3 + + - EMRSA 16 variant phage type PF16d
4 + + - EMRSA 16 PF16a
5 + + - EMRSA 16 PF16a
6 + + - EMRSA 16 PF16a
7 + + - EMRSA 16 PF16a
8 + + - EMRSA 16 variant phage type PF16d
9 (MRSA) control + + - **
1 (MSSA) control + + + **
* no inhibition zone up to 256 米g/ml for all MRSA strains
An external file that holds a picture, illustration, etc.
Object name is 1756-0500-4-458-1.jpg
Figure 1
The effect of pH on the minimum inhibitory concentration of nitrite. The average minimum inhibitory concentrations (MICs) of nitrite for the MSSA control strain (1), a previously typed MRSA control strain (9) and seven MRSA strains (2-8) isolated from the wounds of patients were determined at either pH 4.5, 5.0 or 5.5, using HCl to acidify. The MICs values shown are an average of five independent determinations for each strain and the arrows indicate that pH 4.5 alone was inhibitory to the strains.
An external file that holds a picture, illustration, etc.
Object name is 1756-0500-4-458-2.jpg
Figure 2
The effect of pH on the minimum bactericidal concentration of nitrite. The average minimum bactericidal concentrations (MBC) of nitrite for the seven MRSA strains (2-8) isolated from wounds was compared with the MBC for the MSSA control strain under different pH conditions after either 2 or 24 hours of exposure. HCl was used to acidify the nutrient broth. The error bars represent the standard deviation of the mean MBCs for all seven MRSA strains. At pH 4.5, the maximum concentration of sodium nitrite used in our assay had no effect on the viability of the MSSA strain after 2 hours hence no MBC value is shown.
No adverse effects of the acidified nitrite were reported in this small pilot study.
Discussion
These pilot results highlight the potential efficacy of acidified nitrite as a topical therapy for MRSA. Considering the lack of an effective therapy in this clinical setting it potentially represents a significant therapeutic advance. Wounds are unlikely to become clear of infection spontaneously. Strains isolated from infected wounds showed in vitro sensitivity to acidified nitrite which supports the clinical findings. Indeed, preliminary data suggested that the MRSA strains possibly more sensitive to acidified nitrate than the MSSA strain tested. However, further studies analysing different MSSA strains would be necessary to confirm that this was the case and not due to the individual MSSA strain tested.
Given that there was no difference in the MICs between MRSA isolates from the different subjects, it is unlikely that specific resistance to acidified nitrite could explain failure to clear. Indeed isolate 8 was more sensitive than MSSA in vitro and cleared from only one of two wounds suggesting that local factors were responsible. Failure to clear some infected wounds may be due to recontamination from other infected sites as demonstrated in the patient with renal failure. She cleared at one of 2 sites on the legs and the cleared site was re-infected as was a groin swab after 9 days. In all other wounds that cleared, clinical response was maintained to day nine, four days after stopping treatment. Successful treatment was more common in patients who did not have positive swabs at other sites. Finally, wounds that did not clear of MRSA were more frequently associated with tissue necrosis. Topical therapy may not have penetrated a thick eschar. Better bioavailability may result from doubling the concentrations of acid and nitrite which have been used in the treatment of Buruli ulcers [13].
When acid and nitrite are mixed they react together to release nitric oxide (NO) and nitrous oxide (NO2). These gases may be irritant to the airways and mucosal surfaces so we avoided treating infections of the nose and limited this study to infections on limited "target" areas of the skin and wounds. As NO is a gas with similar physical properties to oxygen, it can diffuse readily into the skin or a wound to treat the infection and does so more readily than conventional antibiotics.
Previous studies in other micro-organisms [12,21] infer that NO is responsible for the effects seen on organisms. Ghaffari demonstrated that exposure to 200 ppm of NO gas for 5 hours was equally bactericidal to S. aureus, MRSA, Escherichia coli, Group B Streptococcus, Pseudomonas aeruginosa, and Candida albicans while NO2 was not effective [22]. This also suggests that NO is responsible for killing the organism. Ghaffari went on to demonstrate that gaseous NO therapy reduced bacterial counts in experimental staphylococcal skin infections without impairing angiogenesis or wound healing [23]. Miller et al exposed cultures of S. aureus to eight sub-lethal exposures of NO in order to select for resistant organisms. However, sensitivity was preserved compared to control organisms exposed only to pure air [24]. The precise mechanism whereby NO is bactericidal is not understood but Martinez's recent study examined staphylococci by electron microscopy and observed cellular oedema after 1 hr exposure, increasing destruction of the cell wall architecture after 7 hours, followed by lysis of cells after 24 hours using a nanoparticle technology to deliver NO [25]. Martinez et al[25] induced wounds in mice and inoculated these with MRSA. Bacteriological burden measured by culture and by gram staining was significantly reduced with 2 applications of NO nanoparticles after 4 and 7 days respectively [25].
The wide range of infections sensitive to acidified nitrite suggest that this would be a useful addition to the prevention and therapy of MRSA and to wound care, especially where several organisms are responsible. There have been no reports of bacteria becoming completely resistant to acidified nitrite, although nitrite has been used for a very long time as a food preservative so organisms have been exposed to this agent.
Limitations of this study
As it was difficult to find subjects meeting the entry criteria of infection confined to a wound, numbers of subjects are small. The study also lacked controls or randomisation to prove that the intervention was responsible for the clearing of infection. Finally the concept of subjects having infection confined to a contaminated wound was likely to be erroneous and standardised swabbing protocols do not exclude carriage of MRSA in the patients' intact skin or immediate environment. Infected wounds that did not clear of MRSA did not have resistant infection but were likely to have been recontaminated or alternatively the bioavailability of acidified nitrite was impaired.
Conclusions
Acidified nitrite is a potential novel therapy for topical application, which can kill MRSA without irritating the skin; this could have important applications in the treatment of localised skin infections, wounds and in the decontamination of hospital staff and merits further study.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3219797/﹛
published: February 2008
The nitrate每nitrite每nitric oxide pathway in physiology and therapeutics
Jon O. Lundberg, Eddie Weitzberg & Mark T. Gladwin Nature Reviews Drug Discovery volume 7, pages156每167(2008)
Key Points
The inorganic anions nitrite (NO2−) and nitrate (NO3−) are usually viewed as inert end products of nitric oxide (NO) metabolism or unwanted residues in the food chain.
Recent studies show that nitrate and nitrite are physiologically recycled in blood and tissue to form NO and other bioactive nitrogen oxides. Thus, they should be viewed as storage pools for NO-like bioactivity, thereby complementing the NO synthase-dependent pathway.
There are two major sources of nitrate and nitrite: the endogenous L-arginine/NO-synthase pathway and the diet. Vegetables are particularly rich in nitrate.
The bioactivation of nitrate from dietary or endogenous sources requires its initial reduction to nitrite, and this conversion is mainly carried out by commensal bacteria inhabiting the gastrointestinal tract.
There are numerous pathways in the body for the further reduction of nitrite to bioactive NO, involving haemoglobin, myoglobin, xanthine oxidoreductase, ascorbate, polyphenols and protons.
The generation of NO by all these pathways is greatly enhanced during hypoxia and acidosis, thereby ensuring NO production in situations for which the oxygen-dependent NO-synthase enzyme activities are compromised.
Nitrite reduction to NO during physiological and pathological hypoxia appear to contribute to physiological hypoxic signalling, vasodilation, modulation of cellular respiration and the cellular response to ischaemic stress.
An expanding number of studies suggest a therapeutic potential for nitrate and nitrite in diseases such as myocardial infarction, stroke, systemic and pulmonary hypertension, and gastric ulceration.
Abstract
The inorganic anions nitrate (NO3−) and nitrite (NO2−) were previously thought to be inert end products of endogenous nitric oxide (NO) metabolism. However, recent studies show that these supposedly inert anions can be recycled in vivo to form NO, representing an important alternative source of NO to the classical L-arginine每NO-synthase pathway, in particular in hypoxic states. This Review discusses the emerging important biological functions of the nitrate每nitrite每NO pathway, and highlights studies that implicate the therapeutic potential of nitrate and nitrite in conditions such as myocardial infarction, stroke, systemic and pulmonary hypertension, and gastric ulceration.
The nitrate每nitrite每nitric oxide pathway in physiology and therapeutics | Nature Reviews Drug Discovery
https://www.nature.com/articles/nrd2466﹛
Published: 02 November 2003
Nitrite reduction to nitric oxide by deoxyhemoglobin vasodilates the human circulationNitrite reduction to nitric oxide by deoxyhemoglobin vasodilates the human circulation Kenyatta Cosby, Kristine S Partovi, Jack H Crawford, Rakesh P Patel, Christopher D Reiter, Sabrina Martyr, Benjamin K Yang, Myron A Waclawiw, Gloria Zalos, Xiuli Xu, Kris T Huang, Howard Shields, Daniel B Kim-Shapiro, Alan N Schechter, Richard O Cannon III & Mark T Gladwin Nature Medicine volume 9, pages1498每1505(2003)
Abstract
Nitrite anions comprise the largest vascular storage pool of nitric oxide (NO), provided that physiological mechanisms exist to reduce nitrite to NO. We evaluated the vasodilator properties and mechanisms for bioactivation of nitrite in the human forearm. Nitrite infusions of 36 and 0.36 米mol/min into the forearm brachial artery resulted in supra- and near-physiologic intravascular nitrite concentrations, respectively, and increased forearm blood flow before and during exercise, with or without NO synthase inhibition. Nitrite infusions were associated with rapid formation of erythrocyte iron-nitrosylated hemoglobin and, to a lesser extent, S-nitroso-hemoglobin. NO-modified hemoglobin formation was inversely proportional to oxyhemoglobin saturation. Vasodilation of rat aortic rings and formation of both NO gas and NO-modified hemoglobin resulted from the nitrite reductase activity of deoxyhemoglobin and deoxygenated erythrocytes. This finding links tissue hypoxia, hemoglobin allostery and nitrite bioactivation. These results suggest that nitrite represents a major bioavailable pool of NO, and describe a new physiological function for hemoglobin as a nitrite reductase, potentially contributing to hypoxic vasodilation.
Nitrite reduction to nitric oxide by deoxyhemoglobin vasodilates the human circulation | Nature Medicine
https://www.nature.com/articles/nm954﹛
Randomized Controlled Trial JAMA Dermatol
. 2015 Aug;
Evaluation of the Efficacy, Safety, and Tolerability of 3 Dose Regimens of Topical Sodium Nitrite With Citric Acid in Patients With Anogenital Warts: A Randomized Clinical Trial
Anthony D Ormerod 1, Pieter C van Voorst Vader 2, Slovomir Majewski 3, Wolfgang Vanscheidt 4, Nigel Benjamin 5, Willem van der Meijden 6
Abstract
Importance: Anogenital warts are a common disorder associated with significant physical and mental distress and a substantial cause of health care costs.
Objective: To assess the efficacy of the topical application of nitric oxide delivered using acidified nitrite.
Design, setting, and participants: A multicenter, randomized, controlled, dose-ranging clinical trial was conducted in European genitourinary medicine clinics between December 20, 2001, and January 14, 2003. Analysis was by intent to treat for all individuals initiating therapy. Participants included male and female volunteers older than 18 years with between 2 and 50 external anogenital warts. A total of 299 individuals from 40 centers were randomized to a control arm and a treatment arm that received 3 doses of acidified nitrite applied topically for 12 weeks with an additional 12 weeks of follow-up, with the final follow-up visit on January 14, 2003.
Interventions: Placebo nitrite cream and placebo citric acid cream were applied twice daily. Active treatment was divided as low dose (sodium nitrite, 3%, with citric acid, 4.5%, creams applied twice daily), middle dose (sodium nitrite, 6%, with citric acid, 9%, creams applied once daily at night, with placebo applied in the morning), and high dose (sodium nitrite, 6%, with citric acid, 9%, creams applied twice daily).
Main outcomes and measures: The primary outcome was proportion of patients with complete clinical clearance of target warts; secondary outcomes were reduction in target wart area and safety.
Results: Complete clinical clearance at 12 weeks occurred in 10 of 74 patients (14%; 95% CI, 6%-21%) with placebo; 11 of 72 (15%; 95% CI, 7%-24%) with low-dose treatment; 17 of 74 (23%; 95% CI, 13%-33%) with middle-dose treatment; and 22 of 70 (31%; 95% CI, 21%-42%) with high-dose treatment (P = .01). Reduction in target wart area, time to clearance, and patient and investigator assessments supported the superiority of the high-dose therapy vs placebo. There were no systemic or serious adverse events associated with treatment. However, there was a dose-related increase in itching, pain, edema, and staining of the anogenital skin associated with the active treatment. Overall, 21 patients withdrew from active treatment because of adverse events compared with none using placebo.
Conclusions and relevance: Use of sodium nitrite, 6%, with citric acid, 9%, twice daily is more effective than placebo in the treatment of anogenital warts. Treatment was associated with local irritant adverse effects.Evaluation of the Efficacy, Safety, and Tolerability of 3 Dose Regimens of Topical Sodium Nitrite With Citric Acid in Patients With Anogenital Warts: A Randomized Clinical Trial - PubMed
https://pubmed.ncbi.nlm.nih.gov/25922903/﹛
Obstet Gynecol . 2008 Jun;
Sinecatechins, a Defined Green Tea Extract, in the Treatment of External Anogenital Warts: A Randomized Controlled Trial
Abstract
Objective:
To estimate the clinical efficacy of topical sinecatechins, a defined green tea extract, in the treatment of external genital and perianal warts.
Methods: This was a randomized, double-blind, vehicle-controlled trial involving 502 male and female patients aged 18 years and older, with 2-30 anogenital warts ranging from 12 to 600 mm(2) total wart area. Patients applied sinecatechins ointment 15% or 10% or vehicle (placebo) three times daily for a maximum of 16 weeks or until complete clearance of all warts, followed by a 12-week treatment-free follow-up to assess recurrence.
Results: Complete clearance of all baseline and newly occurring warts was obtained in 57.2% and 56.3% of patients treated with sinecatechins ointment 15% and 10%, respectively, compared with 33.7% for vehicle (both P<.001). Significance was observed at weeks 4 and 6 and all subsequent visits. Numbers needed to treat were 4.3 and 4.4. Partial clearance rates of at least 50% were reported for 78.4% and 74.0% of patients in the sinecatechins ointment 15% and 10% groups compared with 51.5% of vehicle patients. During follow-up, recurrence of any wart was observed in 6.5%, 8.3%, and 8.8% in the sinecatechins ointment 15% group, sinecatechins ointment 10% group, and vehicle patients, respectively. A total of 3.7%, 8.3%, and 0.0% developed new warts, respectively. A total of 87.7% and 87.3% of patients in the sinecatechins ointment 15% and 10% groups, and 72.1% of vehicle patients experienced application site reactions; 49.2%, 46.2%, and 65.4% of those, respectively, were mild or moderate.
Conclusion: Topical sinecatechins ointments 15% and 10% are effective and well-tolerated in the treatment of anogenital warts.
﹛***
Sinecatechins
- Sinecatechins (USAN, trade names Veregen and Polyphenon E) is an ointment of catechins (55% epigallocatechin gallate) extracted from green tea and other components. It was the first botanical prescription drug approved by the US Food and Drug Administration, in 2006.
Sinecatechins, a Defined Green Tea Extract, in the Treatment of External Anogenital Warts: A Randomized Controlled Trial - PubMed
https://pubmed.ncbi.nlm.nih.gov/18515521/﹛
J Am Acad Dermatol 1998 Apr;
A Randomized Trial of Acidified Nitrite Cream in the Treatment of Tinea Pedis逋悢
R Weller 1, A D Ormerod, R P Hobson, N J Benjamin
Abstract
Background: Nitric oxide is continually released from normal skin and has antimicrobial effects. An acidified nitrite cream releases supraphysiologic concentrations of nitric oxide and is fungicidal in vitro.
Objective: The purpose of this study was to assess the efficacy of an acidified nitrite cream as treatment for tinea pedis.
Methods: Sixty patients were recruited with both a clinical diagnosis of tinea pedis and hyphae identified on direct microscopy; they were randomly placed into an active group treated with twice-daily application of a mixture of 3% salicylic acid in aqueous cream and 3% nitrite in aqueous cream for 4 weeks and a control group treated with 3% salicylic acid in aqueous cream and aqueous cream alone. Nineteen patients completed the trial in the active group and 16 patients in the control group. Mycologic cure (negative results on microscopy and culture) and clinical improvement were measured at 0, 2, and 4 weeks and after a 2-week interval with no treatment.
Results: At the end of the treatment period, 18 of the 19 patients in the active group were mycologically cured as were 11 of 16 in the control group (p = 0.042). Two weeks after the cessation of treatment, 13 of 19 patients in the active group were mycologically cured and 5 of 16 in the control group (p = 0.028). The initial clinical scores in the active and control groups were 8.1 and 8.19 (two-tailed p = 0.95). At 4 weeks they were 1.66 and 6.0 (two-tailed p = 0.002) and after 2 weeks with no treatment 1.45 and 7.4 (two-tailed p < 0.0002).
Conclusion: Acidified nitrite is effective therapy for tinea pedis.
A Randomized Trial of Acidified Nitrite Cream in the Treatment of Tinea Pedis - PubMed
https://pubmed.ncbi.nlm.nih.gov/9555794/﹛
Antimicrobial effect of acidified nitrite on dermatophyte fungi, Candida and bacterial skin pathogens
R. Weller R.J. Price A.D. Ormerod N. Benjamin C. Leifert
Aims: Nitric oxide is generated from sweat nitrite in the acidic environment of the skin surface and is thought to contribute to protection against infection. This study examined the sensitivity of Trichophyton mentagrophytes , T. rubrum , Candida albicans , Streptococcus pyogenes , Staphylococcus aureus and Propionibacterium acnes to acidified nitrite.
Methods and Results: Organisms were cultured in varying concentrations of nitrite and pH for different lengths of time, before being transferred to recovery medium. With the exception of Strep. pyogenes , addition of nitrite increased the antimicrobial activity of acid solutions against all organisms tested. The rank order of sensitivity was: C. albicans < T. rubrum < T. mentagrophytes < Staph. aureus < P. acnes, with P. acnes being most sensitive.
Conclusions: This work has shown that acidified nitrite is microbiocidal to common cutaneous pathogens. The concentrations of nitrite required to kill pathogenic fungi and bacteria in in vitro assays were higher than the concentrations of nitrite measured in sweat. However, additional co坼factors in vivo and in sweat may potentiate the effect of acidified nitrite.
Significance and Impact of the Study: Pharmacological preparations of acidified nitrite are novel antimicrobial agents. These data suggest skin organisms which may be sensitive to this treatment.Antimicrobial effect of acidified nitrite on dermatophyte fungi, Candida and bacterial skin pathogens - Weller - 2001 - Journal of Applied Microbiology - Wiley Online Library
https://sfamjournals.onlinelibrary.wiley.com/doi/full/10.1046/j.1365-2672.2001.01291.x﹛
﹛
Stomach Nitric Oxide
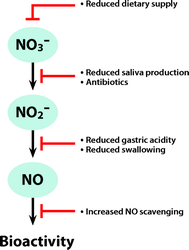
With respect to the known pluripotency of nitric oxide, the high concentrations of nitric oxide normally found in the gastric lumen could be of physiologic importance. High concentrations of nitric oxide are known to be bactericidal,58 and gastric nitric oxide could be a first-line defense against swallowed pathogens. Indeed, in vitro studies have shown that gastric juice and nitrite have markedly better antimicrobial effects on known enteropathogens compared with gastric juice alone.9,59每62 Another proposed role for gastric nitric oxide is in the regulation of mucosal blood flow and mucus production, two important protective mechanisms for gastric mucosal integrity. Application of human saliva rich in nitrite onto rat gastric mucosa ex vivo increases mucosal blood flow and mucus production.63,64 Furthermore, dietary nitrate supplementation in rodents protects the gastric mucosa against ulcerations induced by stress or a nonsteroidal antiinflammatory drug.65,66 Taken together, these findings suggest that nitric oxide and other reactive nitrogen oxides generated from swallowed saliva have several important protective functions to uphold gastric mucosal integrity and to provide a first-line defense against bacterial infection.In this respect, it is highly interesting that sedated and intubated intensive care patients, with poor salivary production and reduced swallowing of saliva and who are often treated with broad-spectrum antibiotics, have virtually abolished gastric nitric oxide (fig. 2).53,67 This nitric oxide can be replenished by gastric administration of nitrite,53 and additional nitrite also increases the circulating concentrations of nitrite in these patients.53 Gastric lesions and bacterial colonization of the gastric lumen is common in the intensive care unit (ICU). In addition, it has been advocated that gastric bacterial colonization could function as a reservoir and later promote ventilator-associated pneumonia. With respect to gastric nitric oxide, the widespread use of H2blockers or proton-pump inhibitors to prevent gastric lesions in the ICU will increase gastric pH, subsequently decreasing stomach nitrite reduction.67 It is tempting to speculate that lack of gastric nitric oxide could partly explain the frequent occurrence of gastric lesions and pneumonia in the ICU. Future studies will reveal whether gastric supplementation with nitrite could have preventive effects in these patients.
Fig. 2. Factors in the intensive care setting that may obstruct the nitrate-nitrite-nitric oxide pathway. Several important steps in nitrate-nitrite-nitric oxide (NO) pathway may be negatively affected in patients treated in the intensive care unit (ICU). The normal dietary intake of nitrate (NO3−, mostly from vegetables) will be almost abolished because both enteral and parenteral feeding formulas contain extremely low concentrations of nitrate and nitrite (NO2−). A patient on full enteral or parenteral feeding is subjected to nitrate/nitrite starvation. Nitrate reduction to nitrite in the oral cavity depends on saliva production and active oral commensal bacteria. Intubated and sedated patients have poor saliva production and are often treated with broad-spectrum antibiotics, which will inhibit this part of the pathway. Normally, swallowed salivary nitrite will immediately be reduced to nitric oxide in the acidic stomach, and this nitric oxide may be involved in host defense and upholding gastric mucosal integrity. ICU patients often have problems swallowing saliva due to sedation and intubation, and their gastric pH is often increased, sometimes pharmacologically, to prevent gastric ulceration. This may partly explain high incidence of gastric ulceration and bacterial colonization found in ICU patients. Finally, many conditions in the ICU are associated with increased oxidative stress in which reactive oxygen species can scavenge nitric oxide, thereby reducing nitric oxide bioactivity.
Antimicrobial Effects of NitriteProfessor, † Postdoctoral Researcher, Section of Anesthesiology and Intensive Care, ‡ Professor, Section of Pharmacology, Department of Physiology and Pharmacology, Karolinska Institutet, Stockholm, Sweden.
Acidification of nitrite results in formation of nitric oxide and other nitrogen oxide species with potent antimicrobial effects against a broad range of potential pathogens.29,62,166 More recently, these antibacterial effects of nitrite have been investigated from a clinical perspective. Yoon et al. used acidified nitrite in an animal model of cystic fibrosis and were successful in clearing the airways of Pseudomonas aeruginosa , a common pathogen in patients with this disease.167
As mentioned above, nitrate is continuously excreted at relatively high concentrations in the urine. During a urinary tract infection, bacteria will reduce nitrate to nitrite, and in the clinic, nitrite test strips are routinely used to indicate an ongoing infection. Nitrite is reduced to nitric oxide and other nitrogen oxide species with potent antibacterial effects, if the urine is mildly acidic (pH 5每6).168 Moreover, nitrite reduction to nitric oxide is greatly potentiated in the presence of the water-soluble and reducing agent, vitamin C.169 It is noteworthy that acidification of urine with different compounds, including vitamin C and cranberry juice, has been used in traditional medicine for prevention and treatment of urinary tract infections.170 In vitro , the antibacterial potency of nitrite and ascorbic acid is comparable with traditional antibiotics.171 The use of indwelling urinary catheters is a major risk factor for catheter-associated urinary tract infection. In spite of optimal care and preventive measures, catheter-associated urinary tract infection is still one of the most common nosocomial infections.172 Carlsson et al. used nitrite and ascorbic acid to generate antibacterial nitrogen species, including nitric oxide in an in vitro model of the urinary bladder.173 By filling the retention balloon of a silicon urinary catheter with these compounds, they were able to generate sufficient amounts of nitric oxide that easily diffused into the surrounding urine. Two different strains of Escherichia coli that were grown in the urine were efficiently killed by this procedure. Later, the same group observed similar in vitro results on a variety of common urinary pathogens in a more advanced flow-through model of urinary tract infection (unpublished data, Eddie Weitzberg, M.D., Ph.D., Department of Physiology and Pharmacology, Karolinska Institute, Stockholm, Sweden, November 2009).Nitrate-Nitrite-Nitric Oxide Pathway:Implications for Anesthesiology and Intensive Care | Anesthesiology | ASA Publications
https://anesthesiology.pubs.asahq.org/article.aspx?articleid=2085806﹛
Case Rep Emerg Med. 2016; 2016: 9013816.
Severe Methemoglobinemia due to Sodium Nitrite Poisoning
Kenichi Katabami, * Mineji Hayakawa, and Satoshi Gando
Abstract
Case. We report a case of severe methemoglobinemia due to sodium nitrite poisoning. A 28-year-old man was brought to our emergency department because of transient loss of consciousness and cyanosis. He was immediately intubated and ventilated with 100% oxygen. A blood test revealed a methemoglobin level of 92.5%. Outcome. We treated the patient with gastric lavage, activated charcoal, and methylene blue (2 mg/kg) administered intravenously. Soon after receiving methylene blue, his cyanosis resolved and the methemoglobin level began to decrease. After relocation to the intensive care unit, his consciousness improved and he could recall ingesting approximately 15 g sodium nitrite about 1 hour before he was brought to our hospital. The patient was discharged on day 7 without neurologic impairment. Conclusion. Severe methemoglobinemia may be fatal. Therefore, accurate diagnosis of methemoglobinemia is very important so that treatment can be started as soon as possible.Introduction
Although methemoglobin levels of >70% are generally fatal, patients with methemoglobin levels of up to 94% have survived [1]. Sodium nitrite intoxication is a common cause of severe methemoglobinemia; however, only one suicidal case has been reported [2]. The concentration of methemoglobin does not exceed 1%-2% in the normal physiological state [3] and levels of 10%每20% generally cause cyanosis. On the other hand, methemoglobin levels of 20%每50% may cause symptoms such as respiratory distress, dizziness, headache, and fatigue. Furthermore, loss of consciousness and death can occur at methemoglobin levels of 50%每70% [4]. Methylene blue is the first choice for treating acute methemoglobinemia. It functions along with natural reduction systems to convert methemoglobin to normal hemoglobin. It is typically administered at doses of 1-2 mg/kg body weight intravenously over 5 min, with symptom improvement expected immediately after the administration. In this report, we describe the successful treatment of a case of severe methemoglobinemia due to sodium nitrite poisoning.Severe Methemoglobinemia due to Sodium Nitrite Poisoning
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4987464/﹛
.png)
.png)