http://www.medscape.com/viewarticle/516241_5
Adipocyte Pseudohypoxia Suppresses Lipolysis and Facilitates Benign Adipose Tissue Expansion
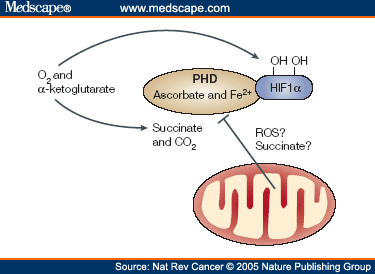
Prolyl hydroxylase enzymes (PHDs) sense cellular oxygen upstream of hypoxia-inducible factor (HIF) signaling, leading to HIF degradation in normoxic conditions. In this study, we demonstrate that adipose PHD2 inhibition plays a key role in the suppression of adipocyte lipolysis. Adipose Phd2 gene ablation in mice enhanced adiposity, with a parallel increase in adipose vascularization associated with reduced circulating nonesterified fatty acid levels and normal glucose homeostasis. Phd2 gene–depleted adipocytes exhibited lower basal lipolysis in normoxia and reduced β-adrenergic–stimulated lipolysis in both normoxia and hypoxia. A selective PHD inhibitor suppressed lipolysis in murine and human adipocytes in vitro and in vivo in mice. PHD2 genetic ablation and pharmacological inhibition attenuated protein levels of the key lipolytic effectors hormone-sensitive lipase and adipose triglyceride lipase (ATGL), suggesting a link between adipocyte oxygen sensing and fatty acid release. PHD2 mRNA levels correlated positively with mRNA levels of AB-hydrolase domain containing-5, an activator of ATGL, and negatively with mRNA levels of lipid droplet proteins, perilipin, and TIP47 in human subcutaneous adipose tissue. Therapeutic pseudohypoxia caused by PHD2 inhibition in adipocytes blunts lipolysis and promotes benign adipose tissue expansion and may have therapeutic applications in obesity or lipodystrophy.脂肪细胞假缺氧抑制脂肪分解,促进良性脂肪组织扩张
Prolyl羟化酶(PHDs)感知缺氧诱导因子(HIF)信号上游的细胞氧,导致常氧条件下的HIF降解。在本研究中,我们证明了抑制脂肪PHD2在抑制脂肪细胞脂解过程中起关键作用。小鼠的脂肪Phd2基因消融术增强了肥胖,与降低循环非酯化脂肪酸水平和正常葡萄糖稳态相关的脂肪血管化平行增加。Phd2基因耗尽脂肪细胞在常氧状态下表现出较低的基础脂肪分解,在常氧状态和缺氧状态下表现出低肾上腺素刺激脂肪分解。一种选择性的PHD抑制剂在小鼠体内和体外抑制小鼠和人脂肪细胞的脂肪分解。PHD2基因消融和药理学抑制降低了关键脂质效应因子激素敏感脂肪酶和脂肪甘油三酯脂肪酶(ATGL)的蛋白水平,提示脂肪细胞氧敏感和脂肪酸释放之间的联系。PHD2 mRNA水平与ATGL激活剂AB-hydrolase domain contain- 5 mRNA水平呈正相关,与人皮下脂肪组织脂滴蛋白、周髂蛋白和TIP47 mRNA水平呈负相关。PHD2抑制脂肪细胞引起的治疗性假缺氧可减少脂肪分解,促进良性脂肪组织扩张,可能在肥胖或脂肪营养不良的治疗中有应用。
PHD2:
http://diabetes.diabetesjournals.org/content/64/3/733
Emerging novel functions of the oxygen-sensing prolyl ...
Oxygen-sensing prolyl hydroxylase domain enzymes (PHDs) target hypoxia-inducible factor (HIF)-α subunits for proteasomal degradation in normoxia through hydroxylation. Recently, novel mechanisms of PHD activation and function have been unveiled. Interestingly, PHD3 can unexpectedly amplify HIF signaling through hydroxylation of the glycolytic enzyme pyruvate kinase (PK) muscle isoform 2 …
JUNE 18 2020
Flavonoids in adipose tissue inflammation and atherosclerosis: one arrow, two targets
Manal Muin Fardoun; Dina Maaliki; Nabil Halabi; Rabah Iratni; Alessandra Bitto; Elias Baydoun; Ali H. Eid Abstract
Flavonoids are polyphenolic compounds naturally occurring in fruits and vegetables, in addition to beverages such as tea and coffee. Flavonoids are emerging as potent therapeutic agents for cardiovascular as well as metabolic diseases. Several studies corroborated an inverse relationship between flavonoid consumption and cardiovascular disease (CVD) or adipose tissue inflammation (ATI). Flavonoids exert their anti-atherogenic effects by increasing nitric oxide (NO), reducing reactive oxygen species (ROS), and decreasing pro-inflammatory cytokines. In addition, flavonoids alleviate ATI by decreasing triglyceride and cholesterol levels, as well as by attenuating inflammatory mediators. Furthermore, flavonoids inhibit synthesis of fatty acids and promote their oxidation. In this review, we discuss the effect of the main classes of flavonoids, namely flavones, flavonols, flavanols, flavanones, anthocyanins, and isoflavones, on atherosclerosis and ATI. In addition, we dissect the underlying molecular and cellular mechanisms of action for these flavonoids. We conclude by supporting the potential benefit for flavonoids in the management or treatment of CVD; yet, we call for more robust clinical studies for safety and pharmacokinetic values.
Flavonoids in adipose tissue inflammation and atherosclerosis: one arrow, two targets | Clinical Science | Portland Press
https://portlandpress.com/clinsci/article-abstract/134/12/1403/225343/Flavonoids-in-adipose-tissue-inflammation-and?redirectedFrom=fulltext
Battling Belly Fat: Specialized Immune Cells Impair Metabolism In Aging
September 28, 2017 ScienceBlog.com
In a new study, Yale researchers have described how nervous systems and immune systems talk to each other to control metabolism and inflammation. Their finding furthers scientists’ understanding of why older adults fail to burn stored belly fat, which raises the risk of chronic disease. The study also points to potential therapeutic approaches to target the problem, the researchers said.
Led by Vishwa Deep Dixit, professor of comparative Medicine and immunobiology, the study was published Sept. 27 in Nature.
Older adults, regardless of body weight, have increased belly fat. However, when they need to expend energy, older people do not burn the energy stored in fat cells as efficiently as younger adults, leading to the accumulation of harmful belly fat. The underlying cause for this unresponsiveness in fat cells was unknown.
In the study, Dixit and his collaborators at Yale, University of Tennessee Health Science Center, and University of Bonn focused on specialized immune cells known as macrophages, which are typically involved in controlling infections. The Dixit lab discovered a new type of macrophage that resides on the nerves in belly fat. These nerve-associated macrophages become inflamed with age and do not allow the neurotransmitters, which are chemical messengers, to properly function.
The researchers also isolated the immune cells from fat tissue of young and old mice, and together with Professor Schultze and his team at the Life and Medical Sciences Institute of the University of Bonn, Germany then sequenced and computationally modelled the genome to understand the problem. “We discovered that the aged macrophages can break down the neurotransmitters called catecholamines, and thus do not allow fat cells to supply the fuel when demand arises,” said Dixit, who is also a member of the Yale Center for Research on Aging.
The researchers found that when they lowered a specific receptor that controls inflammation, the NLRP3 inflammasome, in aged macrophages, the catecholamines could act to induce fat breakdown, similar to that of young mice.
“The key finding is that the immune cells talk to the nervous system to control metabolism,” said Dixit.
In further experiments, the researchers blocked an enzyme that is increased in aged macrophages, restoring normal fat metabolism in older mice. Dixit noted that this enzyme, monoamine oxidase-A or MAOA, is inhibited by existing drugs in the treatment of depression. “Theoretically one could repurpose these MAOA inhibitor drugs to improve metabolism in aged individuals,” he said. But he also cautioned that more research is needed to specifically target these drugs to belly fat and to test the safety of this approach.
In future research, Dixit and his colleagues will further examine the immune cells and their interaction with nerves, and how this neuro-immune dialogue controls health and disease. If controlling inflammation in aging immune cells can improve metabolism, it may have other positive effects on the nervous system or on the process of aging itself, said the researchers.
“The purpose of our research is to achieve greater understanding of immune cell interactions with nerves and fat cells to potentially reduce belly fat, enhance metabolism, and improve performance in the elderly,” said Christina D. Camell, the first author of the study.
Other study authors are Jil Sander, Olga Spadaro, Aileen Lee, Kim Y. Nguyen, Allison Wing, Emily L. Goldberg, Yun-Hee Youm, Chester W. Brown, John Elsworth, Matthew S. Rodeheffer, and Joachim L. Schultze.
The study was supported in part by National Institutes of Health grants, the Glenn Foundation for Medical Research, and Cure Alzheimer’s Fund to Dixit Laboratory.Battling belly fat: Specialized immune cells impair metabolism in aging - ScienceBlog.com
https://scienceblog.com/496653/battling-belly-fat-specialized-immune-cells-impair-metabolism-aging/