静脉滴注抗坏血酸作为一种化学治疗药和生物反应调节剂
Intravenous Ascorbate as a Chemotherapeutic and Biologic Response Modifying Agent
By The Center's Bio-Communications Research Institute
来源:http://brightspot.org/cresearch/intravenousc2.shtml
翻译:蓝山
|
对使用大剂量静脉滴注抗坏血酸(维生素C)这个方案治疗患有糖尿病的癌症病人的卫生保健工作者的重要提示 现已观察到,在接受维生素C静脉滴注后4个小时内,大剂量静脉滴注维生素C(15克或更多)将使血糖试纸在血糖仪读成一个“假阳性”。如果血液从静脉抽取,并在实验室提取血清,就没有这种干扰。因为一些尚未知道的原因,试纸把高水平的抗坏血酸认作是葡萄糖。 请提醒糖尿病病人这种潜在的复杂性。再次重申,它是在试纸上产生的一个“假阳性”——真正的血糖并没有改变。 |
简介
过去的超过30年,我们研究大剂量静脉滴注抗坏血酸(IAA)作为癌症病人的辅助治疗。最初,使用每次15克静脉滴注,每周一次或二次。这些剂量改善病人的幸福感、减轻疼痛以及在许多的病人,延长他们的寿命大大超出肿瘤专家的预言。
12年前,我们使用30克抗坏血酸静脉滴注,每周2次,并且发现,一名男性因原发性肾细胞癌而转移到肺和肝的肿瘤在几周之内消失了。(1) 当时,我们相信IAA帮助癌症病人的机理只是通过二种生物反应调节剂机制:增加细胞外胶原蛋白的合成(正如卡梅隆(Cameron)和鲍林(Pauling)所认为的,“阻隔”肿瘤)以及免疫功能的增强。我们后来报告了一例使用静脉滴注抗坏血酸100克,每周1次或2次,令一名病人的原发性乳腺癌导致的骨转移肿瘤消退(2)。
在另一篇出版的论文(3)我们呈交了抗坏血酸和它的盐(AA)可能不仅仅限于生物反应调节剂的作用的证据。我们发现抗坏血酸对肿瘤细胞有偏好的毒性作用,提示它可以作为一种化学治疗药使用。这种偏好的毒性在体外多种类型的癌细胞中表现出来。我亦提交证据显示需要杀灭癌细胞的抗坏血酸的血清浓度在人体内是可以达到的。其他人已描述过抗坏血酸在体内对多种癌细胞的毒性作用以及动物模型(4-8)。
这里,我们希望总结我们使用IAA治疗大约50名癌症病人的经验。包括我们的方案、注意事项以及2名转移性肾细胞癌病人的病例分析。
治疗的基本原理
从我们的研究(3)我们推断出:
1.因为相对缺乏过氧化物酶,癌细胞更容易受到大剂量、抗坏血酸诱导的过氧化物的攻击;以及
2.可杀灭癌细胞的足够高浓度的抗坏血酸浓度可以在人体内达到。
后来,我们检测接受IAA的病人的血清样本,并且证实了AA浓度可以达到对(体外)癌细胞有毒性的水平。使用密集生长的单层细胞、三维中空纤维肿瘤模型,以及人类血清作为培养基,以模拟发生在体内的环境。我们发现,400 mg/dL的浓度可以杀灭几乎所有类型的癌细胞。最初,我们报告40 mg/dL已经足够(3)。这些早期的资料从体外研究,使用稀疏生长的细胞层,以及标准培养基所得。
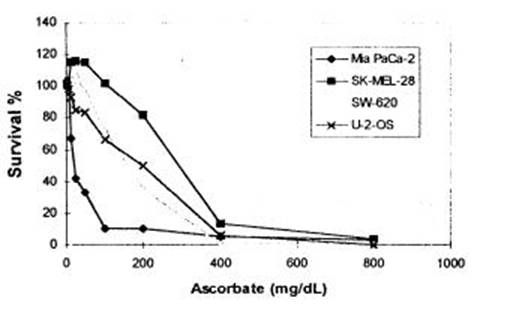
图1 显示在人血清培养基密集生长的4种人类肿瘤细胞系对不断增加剂量的抗坏血酸的反应(

注释:survival%=生存率; Ascorbate=抗坏血酸
图1: Mia PaCa-2(人类胰腺癌)细胞系对抗坏血酸钠(12个样本的平均数)的反应。SK-MEL-28 (人类黑色素瘤), SW-620 (人类结肠癌),以及 U-2-OS (人类骨肉瘤)全部都来自ATCC, Rockville, MD。结果反映所有生存的细胞数。维持培养基是DMEM高葡萄糖培养基(Irvine Sci.),+10%热灭活胎牛血清+抗生素+两性霉素B,5%CO2 湿润培养器,设在摄氏37度。实验培养基分别是来自诊断为各种肿瘤的病人的血清。在加入抗坏血酸钠后培养3天。在96个培养皿种入24000个癌细胞。活细胞的绝对数量使用之前描述的微板块荧光计方法测定(16)。
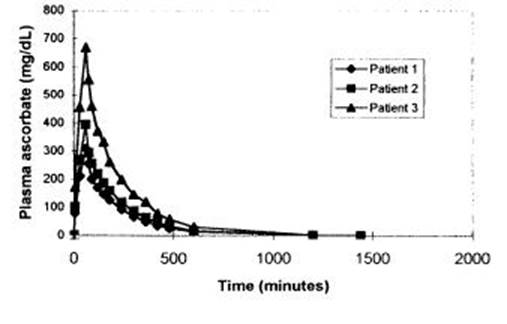
注释: Plasma ascorbate(mg/Dl)=血浆抗坏血酸浓度(mg/Dl); Time(minutes)=时间(分钟)
图2:65克抗坏血酸加入500毫升注射用水,以每分钟1克的速度的静脉滴注过程中的血清抗坏血酸浓度。全血是在接受输液的另一侧手臂的肘前静脉通过静脉留针取得的。血清AA浓度使用高敏感度液体色谱仪测定。患者1(Patient 1)是一名74岁男性,被诊断为没有发生转移的前列腺癌,他在这个研究前的过去的二年接受了超过30次IAA 静脉滴注。患者2(Patient 2)是一名50岁的男性,患有霍金氏淋巴瘤,在这个研究之前接受过16次IAA静脉滴注。患者3(Patient 3)是一名69岁的男性,患有空肠的转移性瘤,在这个研究前接受了16次IAA静脉滴注。
图2描述三个代表性病人给予65克抗坏血酸65分钟后的血清抗坏血酸水平。患者1患有局部性前列腺癌,已经临床治愈。并且在过去曾接受IAA治疗。他取得一个峰值血清浓度702 mg/dL。患者2和3,分别患有非霍金氏瘤和空肠的转移性瘤。在这个检测前,接受过几次IAA静滴,然而,取得更低的血清AA浓度309 mg/dL (患者 3)和396 mg/dL (患者 2)。
从图1和图2的资料,你可以看到,要杀灭癌细胞的所必须的浓度在人的血清至少可以在短时间内取得。图2提示,需要测定静滴IAA后血清抗坏血酸的浓度,以确定病人是否取得预期的足够浓度。
静滴方案
治疗选择
使用IAA治疗癌症绝不应该被视为代替一种已经证明有效的治疗。它只应在以下情况下被考虑:
使用已被证明有效的方法治疗失败的病例;没有已知有效方法治疗的病例;以及
作为对已证明有效的治疗的补充。
因为IAA治疗是一种实验性治疗,一份客观解释的同意书应该由病人阅读、理解并签署。
注意事项和副作用
在我们的实践中,IAA的副作用是罕见的。然而,有些禁忌症和潜在的副作用应该注意:
1. 虽然在文章中只报道过一例,一次单一的10克AA静脉注射导致肿瘤坏死、出血和继发性死亡,正如由卡梅隆(Campbell)和杰克(Jack)(10)所报道的,应该被视为IAA对癌症病人的首要安全考虑。由于这个原因,我们总是从小剂量开始(看静脉滴注部分)。
2. 另一个报告描述一个有双侧输尿管阻塞以及肾功能不全的病人在接受60克IAA治疗后出现的草酸盐肾病(11)。我们亦听讲过一名每天接受IAA治疗的结肠癌病人,出现恶心和呕吐并且因为脱水而住院(12)。二个病例均显示需要保证病人有良好的肾功能、体液充足以及有排尿能力。为此,我们的基本实验室检查包括血清生化学检查以及尿液分析。
3. 出血可能发生在红细胞中葡萄糖-6-磷酸胶氢酶(G6PD)缺乏的病人。我们因此在开始IAA静滴前检测所有病人的G6PD。
4. 静滴部位的局部疼痛可能会出现,如果静滴速度过快。这常常可经减慢速度而纠正。
5. 因为抗坏血酸是一种螯合剂,某些人可能会因为血清钙水平低而出现抽搐。这个可以用缓慢(每分钟1 cc)静脉推注10 cc葡萄糖酸钙治疗。
6.瑞福斯(Rivers)(13)报告,大剂量IAA禁用于肾功能不全、长期血液透析的病人、异常形式的血铁过高以及草酸性结石患者。然而,草酸性结石形成可能被视作一种相对的禁忌症。二个研究小组(14,15)显示,氧化镁(每天口服300毫克)和维生素B6(每天口服10毫克)可以抑制结石患者的草酸结石形成。
7.考虑到作为抗坏血酸载体的液体的数量以及用于调整PH的氢氧化钠/碳酸氢钠的数量,任何对体液或钠增加有不利反应的情况都是相对的禁忌症。比如:阻塞性心力衰竭、腹水、水肿等。
8. 正如任何的静脉滴注部位一样,血管渗透常常是可能的。
9. 抗坏血酸应该只是由静脉滴注。它绝不应该经静脉推注,因为大剂量的同渗重摩可以令外周血管硬化,也不应该由肌肉或皮下注射。经常要衡量液体容量和同渗重摩的问题。我们发现一个小于1200 milliOsmal的同渗重摩(渗透压)可以被大多数病人耐受(表1,可以参看原文件). 。
最基本的准备
在施行大剂量的抗坏血酸前,我们收集以下信息作为一个起始线以及作为一种监测治疗的手段:
血清生化学加上电解质检查
全血计算加上不同血液成分
红细胞G6PD
尿液分析
病人体重
肿瘤类型/分期
特异性血清肿瘤标记物
特异性CT、MRI、骨扫描、以及X-线拍片
表1各种不同数量的抗坏血酸钠/抗坏血酸在注射用水和林格氏乳酸液(mOsm; 等渗= 300 mOsm)的渗透压。高渗混合液加了下画线:使用的混合液从等渗~1200 mOsm,用粗字体表示。在加入浓缩的抗坏血酸钠/抗坏血酸溶液前(500 mg/mL),一个相等容量的IV溶液从袋或瓶中抽取。
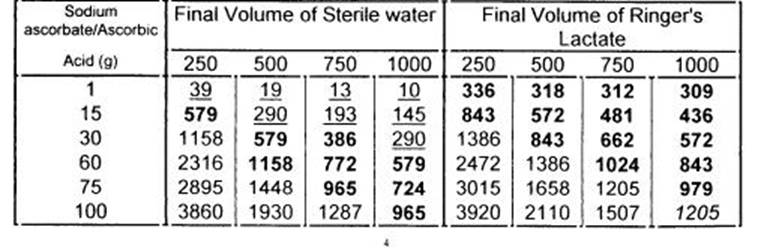
注释:Sodium Ascorbate=抗坏血酸钠; Ascorbic Acid=抗坏血酸; Final Volume of Sterile Water=注射用水最后容量 Final Volume of Ringer’s Lactate= 林格氏乳酸液最后容量
静脉滴注溶液
在大剂量抗坏血酸治疗中,很多静脉滴注溶液是属于高渗的。这并不表示会产生问题,只要滴注速度足够慢以及渗透压不超过1200
milliOsmal (mOsm)。AA的剂量小于15克,我们用林格氏乳酸液(RL)稀释。而更大的剂量,用注射用水稀释。我们现在使用一种抗坏血酸钠/抗坏血酸混合物,每1摩尔抗坏血酸包含0.91摩尔钠(500 毫克 抗坏血酸/毫升, pH 值5.5-7.0, 生产商:
Merit Pharmaceuticals, Los Angeles, California, and Maclaskey Pharmaceuticals,
Wichita, Kansas)。表1显示常用溶液的同渗重摩(渗透压)。
静脉滴注
正如在注意事项指出的, 15克AA,溶于250 mL 格林氏液在1个小时内滴注完毕是建议的一个小的开始剂量。要密切关注病人可能出现的任何不良效果。然后,剂量逐渐增加。滴注速度不应超过每分钟1克AA;大多数人都可以耐受每分钟0.5克。虽然通常会由于日程安排和耐受程度不同而有变化,一个典型的方案将由以下滴注组成:
第一周:每天滴注15克,2-3次/周
第二周:每天滴注30克,2-3次/周
第三周:每天滴注65克,2-3次/周
之后,剂量调整为要取得瞬间400 mg/dL的血清浓度。
根据我们的研究推测,静脉滴注的目标是提高血清抗坏血酸浓度至高于对肿瘤的毒性水平维持尽可能长的时间。因为抗坏血酸不断被肾脏排泄,最佳的滴注速度将会导致抗坏血酸的肿瘤毒性血清水平最长的时间-并且,希望地,最大量的肿瘤细胞杀灭。
我们建议病人每天口服4克抗坏血酸,尤其是在那些没有静脉滴注的日子,以帮助预防可能的坏血病“反弹效应。”
案例史
我们在我们的诊所见过几乎每种类型的实体肿瘤。他们当中的许多接受过IAA,取得不同程度的成功。我们的病例包括胰头癌病人,他只靠IAA作为唯一的治疗,生存了3年半;乳腺癌骨转移和许多非霍金氏淋巴癌的消退;原发性肝癌的消退;转移性结肠癌的消退和缩小;伴有广泛转移的卵巢癌的转移肿瘤的消退和生存期超过3年。我们计划在另一份出版物中公开一份完整整理的病例。
我们只碰到过2例转移性肾细胞癌—一种被认为是无法治疗的疾病。因为结果是如此显著,患上这种疾病的人可能从IAA治疗取得最大的好处。
以下是这二个病例。
病例1
一名52岁的白人女性,有肾细胞癌病史,第一次来到我们的诊所是在1996年10月。
在1995年9月,在她的左肾了发现一个原发性肿瘤后,立即做了一个肾切片检查。组织学证实是肾细胞癌。当时没有发现任何的转移。在1996年3月,X光照片发现肺转移。在1996年9月,一个胸X光片发现她的肺有4个1~3CM的肿块。一个月之后,肺发现有8个1~3CM的肿块(7个在右肺,1个在左肺)。
在她在1996年10月到我们诊所之前,没有接受过任何新的药物、放射或外科治疗。她的起始剂量是15克,二个星期后,增加到65克,每周二次。她亦开始服用:
N-乙酰半胱氨酸(生产商:Vitamin Research Products, Carson City, NV),500 毫克, 口服, 每天一次;
beta-1,3- 葡聚糖(一种巨噬细胞激活剂, 生产商: NSC-24, Nutrition Supply Corp., Carson City, NV), 2.5毫克,每次3片,口服,每天一次;
鱼油(超级EPA, 生产商:Bronson Pharmaceuticals, St. Louis, MO; 含300 mg二十烷王烯酸, 200 mg 二十二碳六烯酸), 1 粒,口服,每天三次;
维生素C, 9克,口服;
beta-胡萝卜素 (Beta Carotene 25, 生产商: Miller Pharmacal Group, Inc., Carol Stream, IL), 每次25,000 单位,口服,每天二次;
L-苏氨酸(生产商:The Solgar Vitamin Co, Inc., Lynbrook, NY), 500毫克,口服每天一次 (纠正实验室检查血清发现的缺乏);
杆状乳酸菌(Lateroflora, International Bio-Tech U.S.A., San Marcos, CA), 280毫克,每次服2片,每天一次。治疗肠道酵母菌/念株菌感染;
复方肌醇烟酸酯 (生产商:Niaplex, Karuna Corp., Novato, CA; 含500 毫克 烟酸, 100 微克 铬)每次2片,口服,每天一次;
以及不含精炼糖的饮食。
她坚持IAA治疗直至1997年6月,此时,胸部X光照片显示原来的8个肿块中的7个已消退,并且第8个亦已缩小。根据医学影像报告,“从前在左肺以及心脏表面上看到的结节性渗透再也看不到了。在左上部看到的结节性渗透显示出明显的区间,体积缩小,同时,只看到大约为1CM大小的模糊阴影。”
病人在1997年6月停止IAA治疗。自此开始,她坚持口服营养性支持方案,而在4年后,她是健康的,没有任何病情进展的证据。
病例2
1985年12月,一名70岁男性老人的右肾下极被发现一个肿块。肿块的病理学检查证实是肾细胞癌。他在另一间诊所由另一名肿瘤专家诊治。大约在手术切除后三个月,病人的X光照片以及CT扫描显示“多发性肺转移以及在他的肝脏发现多处异常的病灶,以及主动脉周围血管免疫母细胞性淋巴结病。”
1986年三月,病人来到我们的诊所求医(1)。他决定不进行化疗。在他的要求下,我们开始给他IAA治疗,30克,每周二次。在1986年4月,在X光照片和CT扫描检查后6周,这个肿瘤专家这样报告,
“…病人重新感觉很好。他的检查结果完全是正常的。他的胸X光照片显示,对比6星期前,肺部的结节有了根本性的好转。主动脉周围血管免疫母细胞淋巴结病已完全消退…,或者是因为从前患过病毒性感染并发肺的病变或者是复发性肾癌,对你的维生素C治疗作出了反应。”
1996年7月的肿瘤学报告指出,“没有任何进行性癌症的证据。他看来健康…今天的胸片提示完全正常。肺结节完全消失了。不管如何,今天没有任何肺转移、肝转移或淋巴结转移的证据。”
在1986年,病人持续接受了每周二次,每次30克静脉滴注治疗达7个月。之后,治疗减少为每周一次,持续8个月。再附加6个月每周二次,每次15克的静脉滴注治疗。在治疗期间和治疗后,病人都没有报告任何毒性反应,以及他的血液生化学检查以及尿液分析都是正常的。病人一直良好,并且定期在我们诊所复查,直至1997年逝去。他去逝时82岁,没有癌症,这是在确诊后的12年。
现在我们的标准方法使用最初的15克,25克和50克静脉滴注。这可以输注达到细胞毒性的足够剂量。
结论
我们相信IAA有作为一种化学治疗药的前景。我们希望,我们的配制和静脉滴注IAA的方法、治疗前、后应该要注意的注意事项,以及临床病例报告,将证明进一步运用IAA对转移性病人进行临床实验和研究是正确的。我们不认为它是对所有癌症都有效的一种治疗。虽然它显示作为一种唯一治疗的效果,尤其是对肾细胞癌,它基本上应该被作为其它有效治疗的一个辅助。
|
by The Center for the Improvement of Human Functioning, International, Inc., Bio-Communications Research Institute. Reprinted with permission. (Emphasis added by DoctorYourself.com editor Andrew Saul) Additional research papers may be read athttp://brightspot.org/cresearch/index.shtml
Introduction Twelve years ago, we used infusions of 30 grams of intravenous ascorbic acid, twice per week, and found that metastatic lesions in the lung and liver of a man with a primary renal cell carcinoma disappeared in a matter of weeks (1). At that time we believed IAA was useful for patients with cancer solely through two biological response modifier mechanisms: increased production of extracellular collagen ("walling off' the tumor as proposed by Cameron and Pauling) and enhancement of immune function. We subsequently reported a case of resolution of bone metastases in a patient with primary breast cancer (1A) using infusions of 100 grams, once or twice per week (2). In a recent publication (3) we presented evidence that ascorbic acid and its salts (AA) could be more than biological response modifiers. We found that ascorbic acid is preferentially toxic to tumor cells suggesting that it could be useful as a chemotherapeutic agent. Preferential toxicity occurred in vitro in multiple tumor cell types. We also presented data suggesting that plasma concentrations of ascorbate required for killing tumor cells were achievable in humans. Others have described in vivo toxicity in multiple tumor types and animal models (4-8). Here we wish to summarize our experience using IAA for approximately 50 patients with cancer. We include our protocol, precautions, and case studies of two patients treated for metastatic renal cell carcinoma.
Treatment rationale Tumor cells are more susceptible to the effects of high-dose, ascorbate-induced peroxidation products because of a relative catalase deficiency; and, Concentrations of ascorbate high enough to kill tumor cells likely can be achieved in humans. Subsequently we tested samples of human serum from patients receiving IAA, and confirmed that AA concentrations can reach levels that are cytotoxic to tumor cells in vitro. Using densely populated monolayers, three-dimensional hollow-fiber tumor models, and human serum as a growth medium to closely mimic what occurs in vivo, we found that an AA concentration of 400 mg/dL effectively kills most tumor cell types. Originally we reported that a concentration of 40 mg/dL was adequate (3). Those early data were generated from in vitro studies using sparsely populated cell monolayers and standard tissue culture medium Figure 1 (which may be seen in the original paper posted at http://brightspot.org/cresearch/intravenousc2.shtml) shows the responses to increasing doses of ascorbate of four human tumor cell lines grown in dense monolayers in a medium of human serum.
Figure 1 Caption:
Figure 2 Caption: Figure 2 depicts plasma ascorbate levels of three representative patients given 65 grams of ascorbate over 65 minutes. Patient 1 with localized prostate cancer was clinically well and had received IAA in the past; he achieved a peak plasma concentration of 702 mg/dL. Patients 2 and 3, had diagnoses of non-Hodgkin's lymphoma, and metastatic carcinoma of the jejunum, respectively. Both had received several IAA infusions at the time of study, yet achieved lower plasma AA concentrations of 309 mg/dL (patient 3), and 396 mg/dL (patient 2). From the data in both Figures 1 and 2, one can see that the concentrations required to kill tumor cells can be achieved at least briefly in human plasma. Figure 2 suggests the need to measure post-IAA plasma ascorbate concentrations to determine if patients are achieving what we expect are adequate concentrations. Infusion Protocol
Treatment choice Cases of treatment failure using proven methods cases with no known effective treatments; and, Cases in which it is used as an adjunct to proven treatments. Because IAA treatment is experimental an appropriate informed consent form should be read, understood, and signed by the patient.
Precautions and side effects 1. Although it has been reported only once in the literature, tumor necrosis, hemorrhage, and subsequent death after a single intravenous 10 gram dose of AA, as reported by Campbell and Jack (10), should be the highest priority concern for the safety of IAA for cancer patients. For this reason, we always begin with a small dose (see Infusion). 2. Another report described acute oxalate nephropathy in a patient with bilateral ureteric obstruction and renal insufficiency who received 60 gram IAA (11). We have also heard one case report of a patient with colon carcinoma, receiving daily IAA, who developed nausea and vomiting and was hospitalized for dehydration (12). Both cases show the need to ensure that patients have adequate renal function, hydration, and urinary voiding capacity. To these ends, our baseline lab tests include a serum chemistry profile and urinalysis. 3. Hemolysis can occur in patients with a red cell glucose-6-phosphate dehydrogenase (G6PD) deficiency. We therefore test G6PD on all patients before beginning IAA infusions. 4. Localized pain at the infusion site can occur if the infusion rate is too high. This is usually corrected by slowing the rate. 5. Because ascorbate is a chelating agent, some individuals may experience shaking due to low serum calcium. This is treated by a slow (1 cc per minute) intravenous push of 10 cc's of calcium gluconate. 6. Rivers (13) reported that high dose IAA is contraindicated in renal insufficiency, chronic hemodialysis patients, unusual forms of iron overload, and oxalate stone formers. However, oxalate stone formation may be considered a relative contraindication. Two groups of researchers (14,15) demonstrated that magnesium oxide (300 rng/d orally) and vitamin B6 (10 mg/d orally) inhibited oxalate stone formation in stone formers. 7. Given the amount of fluid which is used as a vehicle for the ascorbate and the sodium hydroxide/sodium bicarbonate used to adjust the pH, any condition which could be adversely affected by increased fluid or sodium is relatively contraindicated. For example: congestive heart failure, ascites, edema, etc. 8. As with any intravenous site, infiltration is always possible. 9. Ascorbate should only be given by intravenous drip. It should never be given IV push, as the osmolality of high doses are capable of sclerosing peripheral veins, nor should it be given intramuscularly or subcutaneously. There is always a trade-off between fluid volume and osmolality. We have found an osmolality of less than 1200 milliOsmal to be tolerated well by most patients (Table 1, which may be seen in the original paper).
Baseline work-up
Serum chemistry profile with electrolytes
Table 1 Caption:
Infusion solution
Infusion
Week 1: 1 x 15 g infusion per day, 2-3 per
week The dose is then adjusted to achieve transient plasma concentrations of 400 mg/dL, 2-3 infusions per week. According to our working hypothesis, the goal of the infusions is to raise plasma ascorbate concentration above the tumor-cytotoxic level for as long as possible. Because the ascorbate is so readily cleared by the kidney, the optimal infusion rate will result in tumor-cytotoxic plasma levels of ascorbate for the longest time periods--and hopefully, maximum tumor cell killing. We advise patients to orally supplement with 4 grams ascorbate daily, especially on the days when no infusions are made, to help prevent a possible scorbutic "rebound effect."
Case histories We have seen only two cases of metastatic renal cell carcinoma, considered a uniformly untreatable disease. Because the results were so dramatic, people with this disease could potentially benefit the most from IAA treatment. Following are those two cases.
Case 1 She continued IAA treatments until June 1997 when another chest x-ray film revealed resolution of 7 of the 8 masses, and reduction in the size of the 8th. According to the medical imaging report, "The nodular infiltrates seen previously in the right lung and overlying the heart are no longer evident and the nodular infiltrate seen in left upper lung field has shown marked Interval decrease in size and only vague suggestion of an approximately I cm density." The patient discontinued IAA treatments in June 1997. She has continued on an oral nutritional support program since that time, and at this writing (December 1997) is well with no evidence of progression.
Case 2
In March 1986 the patient was seen in our
clinic (1). He decided not to undergo ". . . the patient returns feeling well. His exam is totally normal. His chest x-ray shows a dramatic improvement in pulmonary nodules compared to six weeks ago. The periaortic lymphadenopathy is completely resolved..., either he has had a viral infection with pulmonary lesions with lymphadenopathy that has resolved or (2) he really did have recurrent kidney cancer which is responding to your vitamin C therapy." The oncology report in July 1996 stated, "there is no evidence of progressive cancer. He looks well . . . chest x-ray today is totally normal. The pulmonary nodules are completely gone. There is no evidence of lung metastasis, liver metastasis or lymph node metastasis today, whatsoever." In 1986 the patient received 30 g infusions twice-weekly for 7 months. The treatments were then reduced to once per week for 8 more months. For an additional 6 months he received weekly, 15 g IAA infusions. During and after treatments, the patient reported no toxicities, and his blood chemistry profiles and urine studies were normal. The patient continued well, and was seen periodically at our clinic until early 1997 when he died, cancer-free, at age 82, 12 years after diagnosis.
Conclusion
Support
Neil H. Riordan, PA-C
The Center for the Improvement of Human
Functioning, International, Inc.
Acknowledgments: |
参考书目 REFERENCES
Riordan HD, Jackson JA, Schultz M. Case study: high-dose intravenous vitamin C in the treatment of a patient with adenocarcinoma of the kidney. J Ortho Med 1990;5:5-7.
Riordan N, Jackson JA, Riordan HD. Intravenous vitamin C in a terminal cancer patient. J Ortho Med 1996;11:80-82.
Riordan NH, Riordan HD, Meng X, Li Y, Jackson JA. Intravenous ascorbate as a tumor cytotoxic chemotherapeutic agent. Med Hypodieses 1995;44:207-213.
Cohen MH, Krasnow SH. Cure of advanced Lewis lung carcinoma (LL): A new treatment strategy. Proceedings of AACR 1987;28:416.
Lupulesco A. Vitamin C inhibits DNA, RNA and protein synthesis in epithelial neoplastic cells. Intl J Vit Nutr Res 1991;61:125-129.
Varga JM, Airoldi, L. Inhibition of transplantable melanoma tumor development in mice by prophylactic administration of Ca-ascorbate. Life Sciences 1983;32:1559-1564.
Pierson HF, Meadows GG. Sodium ascorbate enhancement of carbidopa-levodopa methyl ester antitumor activity against pigmented B-16 melanoma. Cancer Res 1983;43:2047-2051.
Chakrabarti RN, Dasgupta PS. Effects of ascorbic acid on survival and cell-mediated immunity in tumor bearing mice. IRCS Med Sci 1984;12:1147-1148.
Tsao CS, Dunham WB, Ping, YL. In vivo antineoplastic activity of ascorbic acid for human mammary tumor. In vivo 1988;2:147-150.
Campbell A, Jack T. Acute reactions to mega ascorbic acid therapy in malignant disease. Scot Med J 1979;24:151.
Wong K, Thomson C, Bailey RR, McDiarmid S, Gardner J. Acute oxalate nephropathy after a massive intravenous dose of vitamin C. Aust NZ J Med 1994:24.
Hanson, J. Personal communication, December 1, 1997.
Rivers JM. Safety of high-level vitamin C ingestion. In: Third Conference on AA. Ann NY Acad Sci 1987;498:95-102.
Rattan V, Sidhu H, Vaidyanathan S, Thind SK, Nath R. Effect of combined supplementation of magnesium oxide and pyridoxine in calcium-oxalate stone formers. UrolRes 1994;22:161-5.
Prien EL, Gershoff S F. Magnesium oxide-pyridoxine therapy for recurrent calcium oxalate calculi. J Urol 1974;l12:509-512.
Riordan HD, Riordan NH, Meng X, Zhong Z, and Jackson JA. Improved microplate fluorometer counting of viable tumor and normal cells. Anticancer Res 1994:927-932.