肿瘤获得维生素C的机制 基质细胞氧化
Stromal Cell Oxidation A Mechanism by Which Tumors Obtain Vitamin C
David B. Agus, Juan C. Vera and David W. Golde, Department of Medicine, Memorial
Sloan-Kettering Cancer Center, New York, New York 10021
DOI: Published September 1999
摘要
人的肿瘤可能含有高浓度的抗坏血酸(Ascorbic
acid),但对它们如何获取维生素知之甚少。某些专门的细胞可以通过抗坏血酸钠共转运蛋白(SVCT)直接转运抗坏血酸,但是在大多数细胞中,维生素C以脱氢抗坏血酸(DHA)的形式通过促进性葡萄糖转运蛋白(GLUT)进入,然后在细胞内被还原并保留为抗坏血酸。研究了已建立造血和上皮细胞异种移植物的小鼠中注射抗坏血酸和脱氢抗坏血酸的积累。大多数造血和上皮肿瘤细胞系只能在体外转运氧化形式的维生素C(DHA);但是,当在小鼠体内作为异种移植物生长时,它们在给予放射性标记的抗坏血酸后迅速积累了维生素C。
GLUTs参与了异种移植肿瘤的维生素C摄取,这是通过D-葡萄糖而非L-葡萄糖的竞争性抑制来证明的。由于恶性细胞不能直接转运抗坏血酸,因此我们认为在肿瘤微环境中抗坏血酸被氧化为脱氢抗坏血酸。共同施用超氧化物歧化酶(SOD)抑制了注射抗坏血酸的动物体内维生素C的肿瘤内蓄积,这暗示了超氧阴离子(Superoxide
anion)在抗坏血酸的氧化中的作用。上皮癌细胞系不能在培养物中产生超氧阴离子,而切碎的异种移植肿瘤却可以。我们的研究表明,GLUTs转运脱氢抗坏血酸(DHA)是肿瘤获取维生素C的一种手段,并表明肿瘤基质细胞产生的超氧阴离子将抗坏血酸氧化,是产生维生素C可运输形式的一种机制。
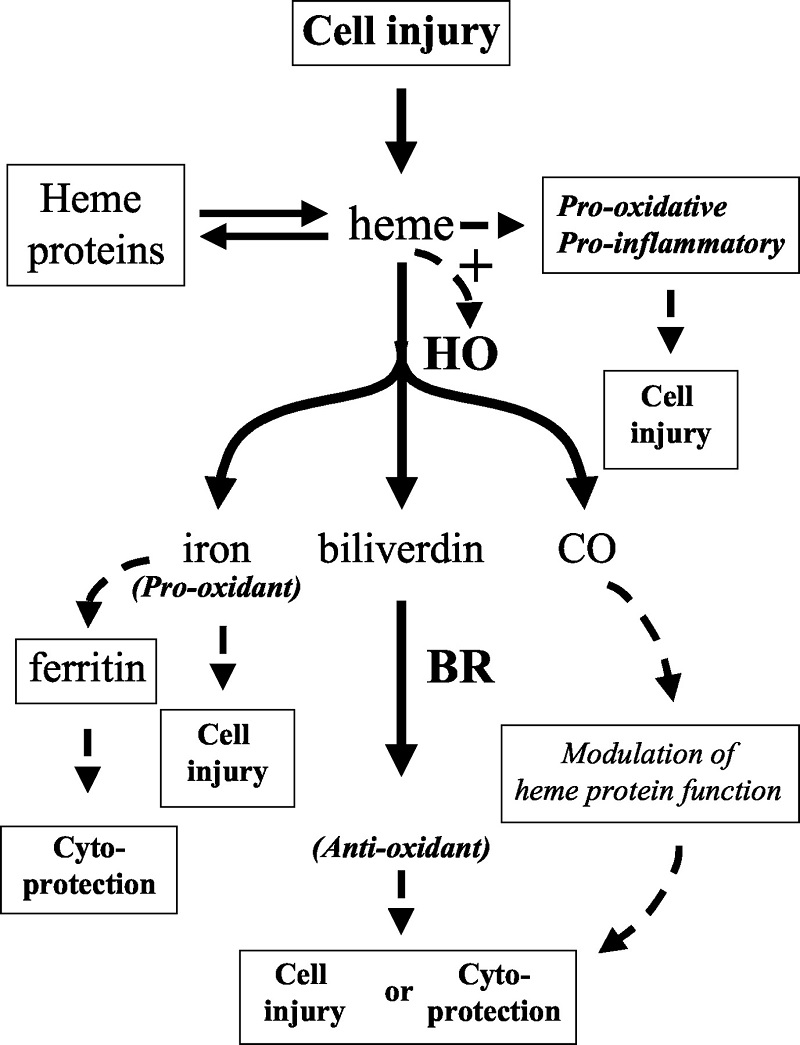
图4所示
提出了维生素C在肿瘤中的转运和积累机制。抗坏血酸(Ascorbic Acid)是血液中维生素C的主要形式,在肿瘤微环境中被超氧阴离子(Superoxide
Anion)氧化为脱氢抗坏血酸(DHA)。因此,氧化是肿瘤转运维生素C(Ascorbic 的一个调节步骤。然后,脱氢抗坏血酸(DHA)通过肿瘤细胞表面的GLUT1转运。然后,肿瘤中的脱
氢抗坏血酸(DHA)被还原为抗坏血酸,由于无法通过GLUT1运输,抗坏血酸被困在肿瘤细胞内。
介绍
饮食中的维生素C对人类和其他灵长类动物至关重要,因为我们无法像大多数其他动物一样在肝脏中合成维生素C(1)。尽管人们对维生素C和维生素C缺乏症的了解很多,但是关于癌症中维生素C的生理学的信息却很少(2)。考虑到维生素C在维持正常免疫过程和宿主防御中的良好作用,人们普遍认为补充维生素C可“增强”免疫系统(3、4)。服用维生素C的癌症患者通常认为它可以增强针对癌症的免疫防御能力。这些观念很少关注癌症本身的营养需求。癌细胞很容易在体外吸收维生素C(5,6),并且研究表明与邻近的正常组织相比,肿瘤中维生素C的浓度较高(7)。然而,肿瘤在体内积累维生素C的机制尚不清楚。
我们先前发现脱氢抗坏血酸(维生素C的氧化形式)在体外通过促进性GLUTs
3(8)转运。爪蟾卵母细胞中GLUT1,GLUT2和GLUT4的表达赋予了其吸收脱氢抗坏血酸的能力,后者在还原为抗坏血酸后保留在细胞内。我们还确定,GLUTs参与正常人嗜中性粒细胞和髓系白血病细胞系HL60的维生素C的运输和积累(8、9、10)。在这些细胞中,脱氢抗坏血酸跨细胞膜运输,并以还原形式抗坏血酸积累,抗坏血酸不可通过双向GLUT(8、9、10)转运。在血脑屏障中脱氢抗坏血酸经GLUT1的运输也是大脑获取维生素C的一种机制(11)。血中维生素C的形式抗坏血酸不易穿过血脑屏障,而脱氢抗坏血酸则穿过血脑屏障并以抗坏血酸的形式积聚在大脑中。
抗坏血酸也可以直接通过最近已被分子表征的Na
+依赖性抗坏血酸转运蛋白(SVCT)家族直接转运(12)。我们没有发现本研究中包括的WBC或前列腺,乳腺和造血肿瘤细胞系中Na
+依赖的抗坏血酸摄取的证据(9,10)。
这项研究表明,前列腺癌,乳腺癌和造血性人类异种移植肿瘤可通过GLUT获得氧化形式的维生素C。肿瘤微环境产生超氧阴离子,将抗坏血酸氧化为脱氢抗坏血酸,即维生素C的可运输形式。通过还原为维生素C的不可运输形式抗坏血酸,可以将运输的脱氢抗坏血酸捕获在肿瘤中。
材料和方法
动物研究。
从国家癌症研究所-弗雷德里克癌症中心获得4至6周大的裸无胸腺BALB /
c雄性小鼠,并经皮下注射。下列浓度的肿瘤细胞进入侧面:HL-60细胞,2.0×107细胞; LNCaP细胞,3.0×106细胞;
MDA468细胞,1.0×107细胞;和CEM细胞为2.0×107。接种后3至4周,可测得约1.0×1.0×1.0
cm肿瘤。将LNCaP细胞以1:1的体积比注入重建的基底膜(Matrigel; Collaborative
Research,贝德福德,马萨诸塞州)。遵循了在研究中正确和人道地使用动物的机构指南。
组织运输和蓄积研究。
将带有异种移植瘤的小鼠的尾静脉注射5μCi的1- [1-14C]抗坏血酸(比活性,6.6 mCi /
mmol;杜邦-NEN,波士顿,马萨诸塞州),[14C]脱氢抗坏血酸或[果糖] -1-3H]蔗糖(比活性,20.0 Ci / mmol;杜邦-NEN)。
[14C]脱氢抗坏血酸是通过将[14C]抗坏血酸与抗坏血酸氧化酶(每1.0
mmol的1-抗坏血酸1个单位,衍生自南瓜属;西格玛化学公司,密苏里州)孵育而产生的。将DTT(0.1 mmol / L;
Sigma)添加到维生素C制剂中。然后解剖器官和肿瘤,并在70%甲醇中匀浆。如先前所述(11),对样品进行闪烁光谱法或HPLC处理。通过添加1mmol / L
EDTA对甲醇馏分进行HPLC。在Whatman强阴离子交换Partisil 10 SAX(4.6×25
cm)色谱柱(Whatman,Inc.,Clifton,NJ)上分离HPLC样品。使用Whatman型WCS溶剂调节柱,洗脱液用System
Gold液相色谱仪(贝克曼仪器公司,加利福尼亚州富勒顿)进行监控,串联连接一个二极管阵列检测器和放射性同位素检测器。通过在265nm处的吸光度和放射性来监测抗坏血酸。脱氢抗坏血酸在265
nm处无吸收,因此只能通过放射性进行监测。用2-脱氧-d-葡萄糖(Sigma),l-葡萄糖(Sigma),SOD(来自人红细胞的超氧化物氧化还原酶;
Sigma)和过氧化氢酶(来自小鼠肝脏的H2O2氧化还原酶; Sigma)进行共注射实验。
超氧阴离子产生的测定
以SOD抑制细胞色素C的还原,测量过氧化物阴离子的产生。将细胞系和新鲜分离的切碎的肿瘤与HBSS在37°C下预孵育15分钟,用含有细胞色素C的HBSS洗涤一次,并在有或没有SOD(200单位/
ml)的HBSS中与1 mg / ml细胞色素C一起孵育。摇表。将PMA(Sigma)以80
nm的最终浓度添加到细胞系中。在60分钟时,将培养基从细胞中移出并置于冰上以终止反应,并立即读取550
nm处的吸光度。细胞色素c的超氧化物特异性还原表示为通过使用21.1
nm-1·cm-1的消光系数在有或没有SOD的情况下孵育的细胞之间的吸光度差异。切碎的肿瘤的细胞计数来自体积测量。
结果
将[14C]抗坏血酸,[14C]脱氢抗坏血酸或[3H]蔗糖到具有异种移植瘤的小鼠注射到尾静脉中,并在注射后1分钟处死。
1分钟后在异种移植组的大脑中发现了大约4%的注射的脱氢抗坏血酸放射活性(表示为每克组织ID的百分比)(图1A)⇓,这一结果与我们之前的研究一致(11)。注射的抗坏血酸和蔗糖在1分钟时仅在大脑匀浆中产生微量放射性,这证实了抗坏血酸不易通过血脑屏障。蔗糖不被代谢或运输,因此被用作血浆容量的标志物(13)。异种移植肿瘤在1分钟时累积注射的脱氢抗坏血酸,LNCaP(前列腺)肿瘤中的〜2.0%ID
/ g肿瘤组织,MDA468(乳腺)肿瘤中的1.6%ID / g,HL-60(骨髓性白血病)的1.2%ID / g
)和CEM(T淋巴细胞白血病;图1A⇓)中的1.2%ID / g。蔗糖未转运到肿瘤中。与大脑不同,将抗坏血酸注入动物体内后,异种移植瘤很容易吸收放射性抗坏血酸。
LNCaP肿瘤组织的肿瘤组织累积量约为2.3%ID / g,MDA-468 1.2%ID / g,HL-60 1.3%ID / g和CEM 1.3%ID /
g(图1A)⇓。进行了来自带有异种移植物的小鼠的肿瘤匀浆的甲醇级分的HPLC分析,以鉴定积累的维生素C的形式。结果表明,在注射脱氢抗坏血酸的动物以及注射抗坏血酸的动物中,肿瘤中积累的维生素C>
86%注射的抗坏血酸(图1B)⇓。
.gif)
图1所示。
维生素C被运输到肿瘤中,而与所注入维生素的氧化还原状态无关。
A,将小鼠用[14C]抗坏血酸,[14C]脱氢抗坏血酸或[3H]蔗糖注射入尾静脉,并在注射后2分钟处死。栏,每组12只动物的平均值;酒吧,东南。
B,注射30μCi的[14C]脱氢抗坏血酸并在1分钟处死的小鼠的异种移植肿瘤(MDA468)的甲醇可溶级分的HPLC分析。在注射了脱氢抗坏血酸(DHA)的动物中,维生素C以抗坏血酸的形式积累(AA;〜91%;保留时间,〜7.93分钟)。
大多数细胞(包括癌细胞)都通过促进性己糖转运蛋白(GLUTs;参考文献8、9、10)以氧化形式将维生素C以脱氢抗坏血酸的形式吸收。因此,抗坏血酸必须在细胞周围环境中被氧化成脱氢抗坏血酸,才能通过GLUT转运到细胞中,然后被还原并以抗坏血酸的形式被捕获。因为大多数肿瘤细胞无法转运抗坏血酸,所以我们假设注射的抗坏血酸在肿瘤微环境中被氧化成脱氢抗坏血酸,然后通过GLUT转运。为了确定抗坏血酸是否以此方式进入肿瘤细胞,我们使用D-葡萄糖和L-葡萄糖进行抑制实验,因为GLUTs选择性转运D-葡萄糖而不是L-葡萄糖。
D-脱氧葡萄糖和D-葡萄糖已显示通过剂量依赖性依赖GLUTs抑制脑中脱氢抗坏血酸的摄取高达70%(11)。在荷瘤小鼠中,D-脱氧葡萄糖抑制了脱氢抗坏血酸和抗坏血酸的转运和积累(图2,A–D)⇓。
5 s时间点用于研究运输,维生素C摄入量(注射的脱氢抗坏血酸和抗坏血酸均)有大约最大35–50%的剂量依赖性抑制。
1分钟和5分钟的时间点代表了运输和蓄积,并显示出约40-65%的剂量依赖性最大程度地抑制了维生素C的吸收(同时注射了脱氢抗坏血酸和抗坏血酸)。施用L-葡萄糖对异种移植物中维生素C的运输或积累没有影响(数据未显示)。在注射抗坏血酸的小鼠中,通过脱氧葡萄糖抑制肿瘤维生素C的摄取和积累表明,抗坏血酸已转化为脱氢抗坏血酸,并由此通过GLUT转运到肿瘤细胞中。

图2所示。
异种移植物中脱氢抗坏血酸通过GLUT1转运的特异性。 [14C]脱氢抗坏血酸和[14C]抗坏血酸进入异种移植肿瘤(A,LNCaP; B,HL-60;
C,CEM;和D,MDA468),并通过增加d-脱氧葡萄糖的量来阻止它们的积累。通过GLUT1(■,0 mg d-脱氧葡萄糖;□,5.3 mg d-脱氧葡萄糖;
Embedded Image,13.4 mg d-脱氧葡萄糖)转运。根据小鼠的平均血容量,其整个动物的基线血糖浓度约为5–7 mm或2.67
mg葡萄糖。在该实验中施用的外源葡萄糖的量是基于小鼠全血糖和随后的倍数以达到最大可耐受量。注射后小鼠中测得的血糖和脱氢抗坏血酸的浓度在注射后的时间内迅速而广泛地变化,因此很难获得有意义的有关血液水平的定量数据。
d-脱氧葡萄糖或l-葡萄糖浓度的增加不会影响血清抗坏血酸和脱氢抗坏血酸的浓度。所有实验均在指定的时间范围内进行。柱,四只小鼠的平均值;柱子,SE。
我们假设抗坏血酸在肿瘤微环境中被超氧阴离子氧化。为了验证这一概念,我们将带有异种移植物的动物与抗坏血酸和SOD,过氧化氢酶或盐水共注射。接受SOD和放射性标记的抗坏血酸的动物的肿瘤维生素C积累减少了约50%,而注射了脱氢抗坏血酸和SOD的动物中维生素C的肿瘤积累没有变化(图3A)⇓。抗坏血酸和过氧化氢酶的共同给药没有效果,表明过氧化物可能在将抗坏血酸氧化为脱氢抗坏血酸中不起作用(图3A)⇓。
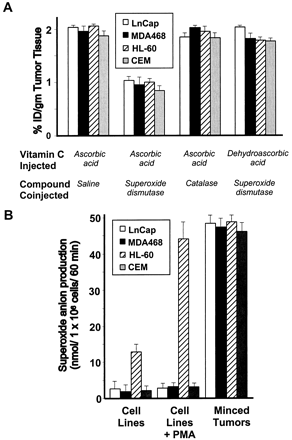
图3所示。
超氧阴离子是肿瘤异种移植物中维生素C氧化的主要原因。A,
[14C]脱氢抗坏血酸和[14C]抗坏血酸均进入肿瘤异种移植物体内,通过异种移植物的抗坏血酸积累被共注射SOD阻断,而不是被过氧化氢酶或生理盐水阻断。所有实验均在1
min.柱上,取4只小鼠的平均值;酒吧、SE。B、肿瘤微环境产生的超氧阴离子是导致肿瘤异种移植物中维生素C氧化的原因。所有实验均超过60分钟。柱,四次重复的平均值;酒吧、SE。
我们测试了肿瘤细胞自身产生超氧阴离子的能力。只有在HL-60细胞系中才能证明超氧阴离子的产生(图3B)⇓,其他细胞系没有能力产生超氧阴离子。如预期的那样,HL-60细胞系在被PMA激活时增加了超氧化物的生成,而其他细胞系则没有显示PMA诱导的超氧化物阴离子的生成。即使是切碎的HL-60异种移植肿瘤,其超氧阴离子生成量也比亲本HL-60细胞系增加了3倍。因为与细胞系不同的切碎的异种移植肿瘤具有产生超氧阴离子的突出能力,因此我们得出结论,肿瘤基质中的非肿瘤细胞是超氧化物产生的原因。
讨论
我们试图通过在裸露的无胸腺小鼠中使用人类肿瘤异种移植模型并测量[14C]抗坏血酸的摄取来确定人类肿瘤细胞如何在体内摄取维生素C。注射抗坏血酸后,肿瘤迅速占据了标记物,尽管我们之前已经在体外证明了细胞无法直接转运抗坏血酸(ascorbic
acid)(8、10)。我们假设肿瘤细胞以脱氢抗坏血酸(DHA)的形式摄取维生素C。观察到的注射脱氧葡萄糖对摄取的抑制作用证实了以下观点:细胞以脱氢抗坏血酸(DHA)的形式获得了通过促进性GLUT转运的维生素C。因为GLUT仅运输氧化形式的维生素C和脱氢抗坏血酸,并且由于细胞没有其他吸收维生素C的机制,所以很明显在肿瘤微环境中抗坏血酸(Ascorbic
Acid)被氧化为脱氢抗坏血酸(DHA)。同时大脑缺乏对14C标签的摄取表明抗坏血酸在循环中没有被氧化。
SOD抑制了注射抗坏血酸的动物体内维生素C的吸收,这表明超氧阴离子(Superoxide
anion)在抗坏血酸的氧化中具有重要作用。上皮肿瘤缺乏超氧阴离子的产生,异种移植肿瘤中超氧阴离子的产生表明基质细胞是超氧阴离子的来源。因此,我们认为通过非恶性基质细胞产生超氧阴离子在肿瘤微环境中氧化抗坏血酸。具有NADPH氧化酶的成纤维细胞,嗜中性粒细胞,单核细胞和内皮细胞已证明产生本构性超氧阴离子(14、15、16、17)。中性粒细胞和HL-60细胞已显示以超氧化物依赖性方式氧化细胞外抗坏血酸(16,18,19)。某些肿瘤(例如,髓样白血病,本研究中的HL-60异种移植物)可以直接氧化抗坏血酸,而其他肿瘤(上皮性肿瘤)则可能依赖于非肿瘤基质细胞来实现此功能。图4
总结了肿瘤对维生素C的转运和积累的模型。

图4所示。
维生素C在肿瘤中运输和积累的机制。抗坏血酸是血液中维生素C的主要形式,在肿瘤微环境中被超氧阴离子氧化为脱氢抗坏血酸。因此,氧化是维生素C肿瘤转移的调控步骤。然后,脱氢抗坏血酸通过GLUT1在肿瘤细胞表面转移。然后,肿瘤中的脱氢抗坏血酸被还原为抗坏血酸,因为它无法通过GLUT1转运,因此被困在肿瘤中。
抗坏血酸钠共转运蛋白存在于许多器官中(12),尽管我们发现该中性粒细胞,单核细胞,HL-60细胞,T淋巴细胞细胞系或前列腺癌和乳腺癌细胞系中没有钠依赖性抗坏血酸摄取。研究(9,10)。因此,通过GLUT以脱氢抗坏血酸的形式摄取维生素C似乎是摄取维生素C的一般机制。如我们先前所显示的,抗坏血酸钠共转运蛋白(SVCT)可能在某些肿瘤的维生素C摄取中起一定作用(6)。
维生素C在肿瘤细胞中的确切功能尚不清楚。研究表明,与正常组织相比,某些人类肿瘤中维生素C的浓度增加(7),并且维生素C对于某些肿瘤细胞的体外生长非常重要(20)。维生素C消耗量的增加会提高血清抗坏血酸的水平(21),因此,可以预期会增加肿瘤中维生素C的浓度。维生素C的细胞内浓度增加可能会影响肿瘤的生长和对肿瘤的反应能力与化学疗法和放射疗法相关的氧化应激。尽管评估维生素C在癌症患者中的作用的研究通常未显示出生存或肿瘤消退方面的益处(22),但尚不清楚人肿瘤中高浓度的维生素C是否为恶性细胞提供了代谢优势。
Stromal Cell Oxidation A Mechanism by Which Tumors Obtain Vitamin C| Cancer Research
https://cancerres.aacrjournals.org/content/59/18/4555
Stromal Cell Oxidation | Cancer Research
https://cancerres.aacrjournals.org/content/59/18/4555
回缩:人胶质瘤细胞对维生素C的摄取超氧阴离子
Retracted: Superoxide‐dependent uptake of vitamin C in human glioma cells
摘要
胶质母细胞瘤是一种致命的脑肿瘤,对目前的细胞抑制治疗无效。维生素C可拮抗活性氧(ROS)生成疗法的作用;然而,尽管它对治疗或肿瘤生长有影响,但它经常被用来减少治疗相关的副作用。由于维生素C在胶质瘤中的摄取机制目前尚不清楚,我们评估了维生素C共转运蛋白(SVCT)和已糖转运蛋白(GLUT)家族在胶质瘤细胞中的表达。此外,由于小胶质细胞可大量浸润高等级胶质瘤(构成胶质母细胞瘤中高达45%的细胞),因此,我们确定了TC620胶质瘤细胞与小胶质样HL60细胞相互作用对维生素C摄取的影响(旁观者效应)。虽然胶质瘤细胞表达了高水平的SVCT亚型2
(SVCT2),但功能活性低,细胞内定位和显性阴性亚型(dnSVCT2)的表达被观察到。胶质母细胞瘤中DHA的主要转运体GLUT - 1
(GLUT1)对2‐脱氧d‐葡萄糖和脱水抗坏血酸(DHA)的摄取率很高,可见胶质瘤细胞葡萄糖代谢活性的增加。共培养胶质瘤细胞和激活的小胶质样HL60细胞导致细胞外抗坏血酸氧化和高DHA被胶质瘤细胞摄取。这种旁观者效应可以解释在高分级胶质瘤中观察到的高抗氧化潜能。
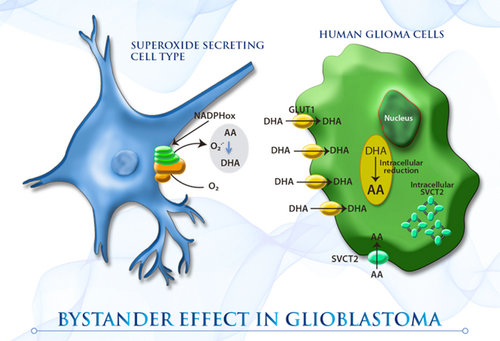
图像
本研究强烈提示,在缺乏功能钠-维生素C协同转运蛋白2
(SVCT2)表达的情况下,胶质瘤细胞与氧化性小胶质细胞的相互作用可能是胶质瘤加载维生素C的重要机制。胶质瘤细胞的高细胞维生素C负荷是由于邻近小胶质细胞产生的细胞外
脱氢抗坏血酸(DHA)被大量摄取所致。考虑到高等级胶质瘤可能是唯一一种产生氧化剂的小胶质细胞数量几乎与肿瘤细胞数量相等的肿瘤,这种旁观者效应可能解释了在高等级胶质瘤中观察到的高抗氧化潜能。
Retracted: Superoxide‐dependent uptake of vitamin C in ...
https://onlinelibrary.wiley.com/doi/full/10.1111/jnc.12365
2003), the Bystander effect induced a staggering 18‐fold increase in vitamin C
uptake by glioma cells after 1 h co‐incubation with activated HL60 cells. In
conclusion, the data from this study indicate that like other tumor cells, human
glioma cells are poorly able to accumulate vitamin C in the form of AA (Fig. 7
), which could be related to the intracellular localization of SVCT2 and the
expression of dnSVCT2.
超极化[1-13C]-抗坏血酸和脱氢抗坏血酸:维生素C作为成像体内氧化还原状态的探针。
Hyperpolarized [1-13C]-ascorbic and dehydroascorbic acid: vitamin C as a
probe for imaging redox status in vivo.
摘要
动态核极化(DNP)的(13)c标记的代谢底物在体外和他们的后续静脉给药允许的位置超极化的底物和它的后续转换成其他代谢产物的动力学在体内被检测。我们报告了[1-(13)C]-抗坏血酸(AA)和[1-(13)C]-去氢抗坏血酸(DHA)的超极化,以及维生素C的还原和氧化形式,并评价了它们作为肿瘤氧化还原状态探针的性能。两种制剂在pH值为3.2时,溶液态极化率为10.5±1.3%,而在pH值为7.0时,[1-(13)C]-
aa的极化率为5.1±0.6%,[1-(13)C]- dha的极化率为8.2±1.1%。标记核的自旋晶格弛豫时间(T(1) s)长为9.4 T:
AA为15.9±0.7 s, DHA为20.5±0.9 s。在小鼠淋巴瘤细胞悬浮液中观察到细胞外氧化[1-(13)C]- aa和细胞内还原[1-(13)C]-
dha。体外监测了DHA与细胞抗氧化剂谷胱甘肽的自发反应,其速率比细胞悬浮液中观察到的速率低约100倍,表明酶参与了细胞内的还原。[1-(13)C]在体内淋巴瘤肿瘤中也检测到dha减少。相反,在相同的肿瘤中没有检测到氧化[1-(13)C]-
aa,这与肿瘤维持一个缩小的微环境的观点一致。本研究表明,超极化(13)C标记的维生素C可作为体内氧化还原状态的非侵入性生物标志物,具有转化为临床应用的潜力。
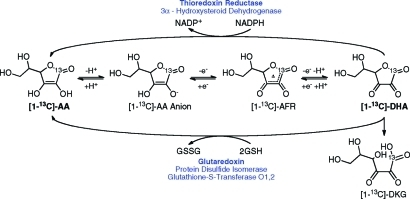
图1:图中突出显示了维生素c在细胞内主要的氧化、还原和降解途径,图中显示了谷胱甘肽和nadph依赖的还原脱氢抗坏血酸(DHA)的酶,其中主要的胞内酶用粗体表示。抗坏血酸自由基(亦称半氢化抗坏血酸);DHA,脱氢抗坏血酸;DKG
diketogulonic酸;谷胱甘肽,谷胱甘肽减少;GSSG氧化谷胱甘肽。
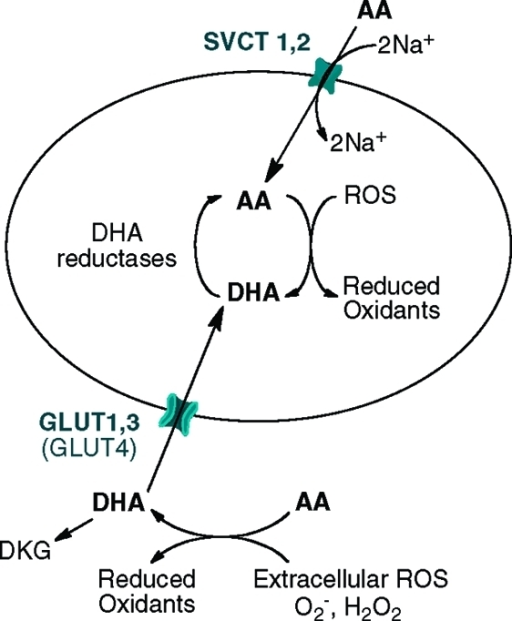
图2:钠依赖的维生素C转运蛋白1和2 (SVCT 1和2)以及葡萄糖转运蛋白(主要是GLUT1、3和GLUT
4)分别以还原(AA)和氧化(DHA)两种形式运输维生素C的机制。这是由DHA的GLUT1转运在癌细胞中占主导地位,由于肿瘤细胞外的酸性pH值,SVCTs要么不表达,要么活性低.
结论:在小鼠淋巴瘤细胞悬浮液中,观察到细胞外氧化[1-(13)C]- aa和细胞内还原[1-(13)C]-
dha。体外监测了DHA与细胞抗氧化剂谷胱甘肽的自发反应,其速率比细胞悬浮液中观察到的速率低约100倍,表明酶参与了细胞内的还原。[1-(13)C]在体内淋巴瘤肿瘤中也检测到DHA减少。本研究表明,超极化(13)C标记的维生素C可作为体内氧化还原状态的非侵入性生物标志物,具有转化为临床应用的潜力。
维生素C的简化型,抗坏血酸(AA),是一个重要的水溶性抗氧化剂和许多酶的辅因子参与生物合成的反应。(14)AA是可逆氧化(图1),形成了稳定的抗坏血自由基(误判率)中间失去一个电子或脱氢抗坏血酸(DHA)的损失两个电子。误判率衰减通过歧化AA和DHA,而DHA自发水解在生理条件下,与内酯的开环形成diketogulonic酸(DKG)。(15),而肠的上皮细胞,肝脏和肾脏可以直接通过运输AA
钠-依赖维生素C转运蛋白(SVCT1
2(16)),大多数组织获得维生素C的主要手段是通过运输DHA,在水溶液中形成稳定的双环水合半缩醛结构。17、18肿瘤细胞系的研究发现,他们要么缺乏能力运输AA,或者如果他们有能力,DHA的吸收速度至少快一个数量级。(19)DHA被葡萄糖
转运蛋白13,
20 GLUT1(Km= 1.1mM)和GLUT3(Km= 1.7mM)(图2),(21)这一现象被广泛应用于[18F]-氟脱氧葡萄糖正电子发射断层扫描(FDG
PET)对肿瘤的检测和分期。一旦进入细胞,DHA必须迅速还原以避免水解(23)并保持抗氧化活性。这可以发生自发或酶,反应与谷胱甘肽(GSH)或GSH-依赖酶(Km=
2.2mM)和通过NADPH-依赖反应,主要涉及酶硫氧还蛋白还原酶(Km= 0.7mM)。(14)包括双硫键谷胱甘肽依赖酶氧化还原酶,glutaredoxin蛋白二硫化物异构酶,而主要NADPH-依赖酶是硒蛋白,硫氧还蛋白还原酶(图1)。在这两种情况下,细胞内DHA的快速减少将保持一个良好的转运梯度。
Abstract
Dynamic nuclear polarization (DNP) of (13)C-labeled metabolic substrates in
vitro and their subsequent intravenous administration allow both the location of
the hyperpolarized substrate and the dynamics of its subsequent conversion into
other metabolic products to be detected in vivo. We report here the
hyperpolarization of [1-(13)C]-ascorbic acid (AA) and [1-(13)C]-dehydroascorbic
acid (DHA), the reduced and oxidized forms of vitamin C, respectively, and
evaluate their performance as probes of tumor redox state. Solution-state
polarization of 10.5 ± 1.3% was achieved for both forms at pH 3.2, whereas at pH
7.0, [1-(13)C]-AA retained polarization of 5.1 ± 0.6% and [1-(13)C]-DHA retained
8.2 ± 1.1%. The spin-lattice relaxation times (T(1)'s) for these labeled nuclei
are long at 9.4 T: 15.9 ± 0.7 s for AA and 20.5 ± 0.9 s for DHA. Extracellular
oxidation of [1-(13)C]-AA and intracellular reduction of [1-(13)C]-DHA were
observed in suspensions of murine lymphoma cells. The spontaneous reaction of
DHA with the cellular antioxidant glutathione was monitored in vitro and was
approximately 100-fold lower than the rate observed in cell suspensions,
indicating enzymatic involvement in the intracellular reduction. [1-(13)C]-DHA
reduction was also detected in lymphoma tumors in vivo. In contrast, no
detectable oxidation of [1-(13)C]-AA was measured in the same tumors, consistent
with the notion that tumors maintain a reduced microenvironment. This study
demonstrates that hyperpolarized (13)C-labeled vitamin C could be used as a
noninvasive biomarker of redox status in vivo, which has the potential to
translate to the clinic.
Bottom Line: Extracellular oxidation of [1-(13)C]-AA and intracellular
reduction of [1-(13)C]-DHA were observed in suspensions of murine lymphoma
cells.The spontaneous reaction of DHA with the cellular antioxidant glutathione
was monitored in vitro and was approximately 100-fold lower than the rate
observed in cell suspensions, indicating enzymatic involvement in the
intracellular reduction. [1-(13)C]-DHA reduction was also detected in lymphoma
tumors in vivo.This study demonstrates that hyperpolarized (13)C-labeled vitamin
C could be used as a noninvasive biomarker of redox status in vivo, which has
the potential to translate to the clinic.
Mentions
The reduced form of vitamin C, ascorbic acid (AA), is an essential water-soluble
antioxidant and cofactor of numerous enzymes involved in biosynthetic
reactions.(14) AA is reversibly oxidized (Figure 1), forming the stable ascorbic
free radical (AFR) intermediate with the loss of one electron or dehydroascorbic
acid (DHA) with the loss of two electrons. AFR decays by disproportionation to
AA and DHA, whereas DHA spontaneously hydrolyses under physiological conditions,
with the opening of the lactone ring to form diketogulonic acid (DKG).(15) While
epithelial cells of the intestine, liver, and kidney can transport AA directly
through sodium-dependent vitamin C transporters (SVCT1, 2(16)), the primary
means by which most tissues acquire vitamin C is through transport of DHA, which
forms a stable bicyclic hydrated hemiacetal structure in aqueous solution.17,18
Studies in tumor cell lines have found that they either lack the capacity to
transport AA, or if they do have the capability, the rate of uptake of DHA is at
least an order of magnitude faster.(19) DHA is taken up by the glucose
transporters13,20 GLUT1 (Km = 1.1 mM) and GLUT3 (Km = 1.7 mM) (Figure 2), which
display a similar affinity for both glucose and DHA and are overexpressed in
most tumors compared to normal tissue.(21) This phenomenon is widely exploited
in detection and staging of tumors by [18F]-fluorodeoxyglucose positron emission
tomography (FDG PET).9,22 Once inside the cell, DHA must be reduced rapidly to
avoid hydrolysis(23) and maintain antiscorbutic activity. This can occur
spontaneously or enzymatically, by reaction with glutathione (GSH) or
GSH-dependent enzymes (Km = 2.2 mM) and through NADPH-dependent reactions,
primarily involving the enzyme thioredoxin reductase (Km = 0.7 mM).(14)
Glutathione-dependent enzymes include the thiol-disulfide oxidoreductases,
glutaredoxin and protein disulfide isomerase, while the predominant
NADPH-dependent enzyme is the selenoprotein, thioredoxin reductase (Figure 1).
In both cases, rapid intracellular reduction of DHA will maintain a favorable
gradient for transport.(19)
Mechanism of vitamin C transport, in both reduced (AA) | Open-i
https://openi.nlm.nih.gov/detailedresult?img=PMC3144679_ja-2011-045925_0002&req=4
Department of Biochemistry, University of Cambridge
Bohndiek SE, Kettunen MI, Hu DE, Kennedy BW, Boren J, Gallagher FA, Brindle KM -
Journal of the American Chemical Society (2011)
维生素C是一种激酶抑制剂:脱氢抗坏血酸抑制IκBα激酶β,因此NF-kB的激活
Vitamin C Is a Kinase Inhibitor: Dehydroascorbic Acid
Inhibits IκBα Kinase β
Department of Clinical Laboratories,1 Program in Molecular
Pharmacology and Chemistry, Memorial Sloan-Kettering Cancer Center,2 Structural
Biology Program, Department of Physiology and Biophysics, Mount Sinai School of
Medicine, New York, New York3
活性氧(ROS)是细胞信号转导通路中的关键中间体,其功能可能会被抗氧化剂抵消。抗坏血酸(AA)充当抗氧化剂,提供两个电子并被氧化成脱氢抗坏血酸(DHA)。我们发现DHA在体外直接抑制IκBα激酶β(IKKβ)和IKKα的酶活性,而AA则没有这种作用。当在细胞中加载AA并通过表达组成型活性IKKβ的细胞中的氧化应激诱导生成DHA时,NF-κB激活受到抑制。我们的结果确定了维生素C在信号转导中的双重分子作用,并提供了维生素C的氧化还原状态与NF-κB信号事件之间的直接联系。
AA淬灭参与NF-κB活化的ROS中间体,并被氧化成DHA,直接抑制IKKβ和IKKα的酶活性。这些发现定义了维生素C在信号传导中的功能,除了作为抗氧化剂外,还从机理上阐明了维生素C如何下调NF-κB信号传导。
我们调查了ROS的细胞内生成器,例如二甲氧萘并萘醌(DMNQ),是否还会抑制预加载了2.5 mM维生素C的细胞中的IKKβ(SS /
EE)依赖性荧光素酶活性。DMNQ的浓度对荧光素酶活性或发现用IKKβ(SS / EE)转染的细胞的生存力为5μM。装有2.5
mM维生素C并与5μMDMNQ孵育的细胞显示萤光素酶活性显着降低(P = 0.003,n =
3,单尾t检验)(图(图6B).6B)。这些结果表明,AA向DHA的细胞内转化抑制了IKKβ(SS /
EE)的激酶活性,表明DHA在体内起激酶抑制剂的作用。我们认为,加载维生素C并置于氧化条件下的细胞中,AA可以抑制ROS并转化为DHA。生成的DHA可以通过促进性葡萄糖转运蛋白离开细胞,或作为直接激酶抑制剂发挥作用,如此处所示,用于IKKα和IKKβ(图(图7).7)。这些过程通过抗氧化剂维生素C将氧化应激与激酶抑制联系起来。

图。 7。
维生素C调节信号响应的示意图。维生素C作为DHA通过葡萄糖转运蛋白进入细胞,并迅速还原为AA。
ROS通过激活IKKβ诱导NF-κB信号传导应答,而AA猝灭ROS,抑制IKKβ的激活。在这些过程中,AA氧化成DHA,而DHA抑制IKKβ。
Vitamin C Is a Kinase Inhibitor: Dehydroascorbic Acid Inhibits IκBα Kinase β
Reactive oxygen species (ROS) are key intermediates in cellular signal
transduction pathways whose function may be counterbalanced by antioxidants.
Acting as an antioxidant, ascorbic acid (AA) donates two electrons and becomes
oxidized to dehydroascorbic acid (DHA). We discovered that DHA directly inhibits
IκBα kinase β (IKKβ) and IKKα enzymatic activity in vitro, whereas AA did not
have this effect. When cells were loaded with AA and induced to generate DHA by
oxidative stress in cells expressing a constitutive active IKKβ, NF-κB
activation was inhibited. Our results identify a dual molecular action of
vitamin C in signal transduction and provide a direct linkage between the redox
state of vitamin C and NF-κB signaling events. AA quenches ROS intermediates
involved in the activation of NF-κB and is oxidized to DHA, which directly
inhibits IKKβ and IKKα enzymatic activity. These findings define a function for
vitamin C in signal transduction other than as an antioxidant and
mechanistically illuminate how vitamin C down-modulates NF-κB signaling.
We investigated whether an intracellular generator of ROS, such as
dimethoxinaphthoquinine (DMNQ), would also inhibit IKKβ(SS/EE)-dependent
luciferase activity in cells preloaded with 2.5 mM vitamin C. The concentration
of DMNQ that had no effect on luciferase activity or viability in cells
transfected with IKKβ(SS/EE) was found to be 5 μM. Cells loaded with 2.5 mM
vitamin C and incubated with 5 μM DMNQ showed significantly reduced luciferase
activity (P = 0.003, n = 3, one-tailed t test) (Fig. (Fig.6B).6B). These
results indicate that intracellular conversion of AA to DHA inhibits kinase
activity of IKKβ(SS/EE), suggesting that DHA functions as a kinase inhibitor in
vivo. We propose that in cells loaded with vitamin C and placed under oxidative
conditions, AA quenches ROS and transforms into DHA. The DHA generated can leave
the cell via facilitative glucose transporters or function as a direct kinase
inhibitor as shown here for IKKα and IKKβ (Fig. (Fig.7).7). These processes
link oxidative stress to kinase inhibition via the antioxidant, vitamin C.
Vitamin C Is a Kinase Inhibitor: Dehydroascorbic Acid Inhibits IκBα Kinase β
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC444845/
Vitamin C Is a Kinase Inhibitor: Dehydroascorbic Acid Inhibits IκBα Kinase β
| Molecular and Cellular Biology
https://mcb.asm.org/content/24/15/6645
脱氢抗坏血酸的新型生物学作用:抑制Na +依赖性抗坏血酸的转运
A novel biological role of dehydroascorbic acid: Inhibition
of Na+-dependent transport of ascorbic acid
Dipartimento di Scienze Biomolecolari, Università degli Studi di Urbino
“Carlo Bo”, Urbino 61029, Italy
使用U937细胞克隆,以相同的速率吸收低微摩尔浓度的抗坏血酸(AA)和脱氢抗坏血酸(DHA),用于研究介导两种形式维生素的细胞吸收的转运系统之间可能的相互作用。用不同的实验方法获得的结果表明,DHA有效且可逆地抑制了Na
+
-AA共转运蛋白对AA的吸收。因此,在固定量的AA存在下,细胞外DHA浓度的逐渐升高会导致维生素C净积累量的初始下降,最终,在较高水平下,仅基于DHA摄取会导致维生素的积累通过己糖转运蛋白。在其他各种细胞类型中也检测到了DHA依赖的AA吸收抑制作用。综上所述,我们的结果提供了由与炎症部位产生的DHA浓度相适应的DHA浓度介导的新型生物学效应的证据。
.jpg)
A novel biological role of dehydroascorbic acid: Inhibition of Na+-dependent
transport of ascorbic acid
A U937 cell clone, in which low micromolar concentrations of ascorbic acid (AA)
and dehydroascorbic acid (DHA) are taken up at identical rates, was used to
investigate possible interactions between transport systems mediating cellular
uptake of the two forms of the vitamin. Results obtained with different
experimental approaches showed that DHA potently and reversibly inhibits AA
uptake through Na+-AA cotransporters. Hence, a progressive increase in
extracellular DHA concentrations in the presence of a fixed amount of AA caused
an initial decrease in the net amount of vitamin C accumulated, and eventually,
at higher levels, it caused an accumulation of the vitamin solely based on DHA
uptake through hexose transporters. DHA-dependent inhibition of AA uptake was
also detected in various other cell types. Taken together, our results provide
evidence of a novel biological effect mediated by concentrations of DHA
compatible with those produced at inflammatory sites.
A novel biological role of dehydroascorbic acid: Inhibition of Na+-dependent
transport of ascorbic acid - ScienceDirect
https://www.sciencedirect.com/science/article/abs/pii/S1043661814000462
黄酮类化合物阻断脱氢抗坏血酸(DHA)和抗坏血酸(AA)的摄取,抑制细胞内AA和DHA在积累U937和Jurkat细胞中的积累
Intracellular Accumulation of Ascorbic Acid Is Inhibited by
Flavonoids via Blocking of Dehydroascorbic Acid and Ascorbic Acid Uptakes in
HL-60, U937 and Jurkat Cells
马克·莱文(James B.
营养学杂志,第130卷,第5期,2000年5月,第1297页
抽象
在HL-60,U937和Jurkat细胞中,细胞内抗坏血酸的积累是通过摄取脱氢抗坏血酸(抗坏血酸的氧化代谢产物)和抗坏血酸(维生素C)而发生的。脱氢抗坏血酸和抗坏血酸分别通过钠依赖性葡萄糖转运蛋白(GLUT
1和GLUT 3)和钠依赖性抗坏血酸转运蛋白转运到细胞中。
类黄酮通过阻断转化细胞中的脱氢抗坏血酸和抗坏血酸的摄取来抑制细胞内抗坏血酸的积累。当类黄酮浓度为10-70μmol/
L时,细胞中约50%的脱氢抗坏血酸摄取被抑制。在Jurkat细胞中,两种有效的类黄酮(杨梅素和槲皮素)竞争性地抑制脱氢抗坏血酸的摄取,Ki值分别约为14和15μmol/
L。因为GLUT 1和GLUT 3转运脱氢抗坏血酸,所以使用过表达大鼠GLUT 1或人GLUT
3的中国仓鼠卵巢细胞研究了黄酮类化合物对脱氢抗坏血酸摄取的抑制作用。杨梅素分别抑制22和18μmol/ L的浓度在过表达GLUT 1和GLUT
3的细胞中脱氢抗坏血酸的摄取。杨梅素还抑制抗坏血酸的摄取。在Jurkat细胞中,Ki = 14μmol/ L时,抑制作用是非竞争性的。
这些数据表明类黄酮抑制抗坏血酸和脱氢抗坏血酸的摄取,但是通过不同的机制抑制。这些数据可能有助于对类黄酮对人体细胞中抗坏血酸细胞内积累的生物学作用的新认识。
Intracellular Accumulation of Ascorbic Acid Is Inhibited by Flavonoids via
Blocking of Dehydroascorbic Acid and Ascorbic Acid Uptakes in HL-60, U937 and
Jurkat Cells
Jae B. Park, Mark Levine
The Journal of Nutrition, Volume 130, Issue 5, May 2000, Pages 1297–
ABSTRACT
In HL-60, U937 and Jurkat cells, the intracellular accumulation of ascorbic acid
occurred via uptakes of both dehydroascorbic acid (an oxidized metabolite of
ascorbic acid) and ascorbic acid (vitamin C). Dehydroascorbic acid and ascorbic
acid were transported into cells by sodium-independent glucose transporters
(GLUT 1 and GLUT 3) and sodium-dependent ascorbic acid transporters,
respectively.
Flavonoids inhibited the intracellular accumulation of ascorbic acid by
blocking dehydroascorbic acid and ascorbic acid uptakes in the transformed
cells.
At flavonoid concentrations of 10–70 μmol/L, ∼50% of dehydroascorbic acid
uptake was inhibited in the cells. In Jurkat cells, two potent flavonoids
(myricetin and quercetin) competitively inhibited dehydroascorbic acid uptake,
and Ki values were ∼14 and 15 μmol/L, respectively. Because GLUT 1 and GLUT 3
transport dehydroascorbic acid, the inhibition of dehydroascorbic acid uptake by
flavonoids was investigated by using Chinese hamster ovary cells overexpressing
rat GLUT 1 or human GLUT 3. Myricetin at concentrations of 22 and 18 μmol/L,
respectively, inhibited half of dehydroascorbic acid uptake in the cells
overexpressing GLUT 1 and GLUT 3. Myricetin also inhibited ascorbic acid uptake;
inhibition was noncompetitive with Ki = 14 μmol/L in Jurkat cells.
These data indicate that flavonoids inhibit both ascorbic acid and
dehydroascorbic acid uptake but do so by different mechanisms. These data may
contribute to new understanding of the biological effect of flavonoids on the
intracellular accumulation of ascorbic acid in human cells.
Reduction of dehydroascorbic acid by homocysteine
Author links open overlay panelJae BPark
Phytonutrients Laboratory, Bldg. 307, Rm. 313, BHNRC, ARS, USDA, Beltsville, MD
20705, USA
Abstract
To determine the reductive process of extracellular dehydroascorbic acid (DHA),
molecules (homocysteine, homocysteine thiolactone, methionine, cysteine, and
homoserine) were tested to identify those with the potential to reduce DHA to
ascorbic acid (AA). Homocysteine (Hcy) was the most potent of the molecules
tested. The efficacy of Hcy was compared with that of other molecules able to
reduce DHA (reduced glutathione (GSH) and cysteine (Cy)). Although all three
molecules were able to reduce DHA, GSH and Cy were not to reduce DHA to AA at
concentrations lower than 100 μmol/l, and only less than 5% DHA was reduced to
AA at concentrations of 200–300 μmol/l. In contrast, Hcy reduced DHA to AA
stoichiometrically at concentrations as low as 10 μmol/l. In Jurkat and U937
cells, the increasing concentrations of extracellular Hcy suppressed
intracellular dehydroascorbic acid uptake, indicating that extracellular
reduction of DHA by Hcy leads to decreasing extracellular DHA available for its
intracellular uptake. Simultaneous oxidation and reduction of Hcy and DHA were
accelerated extracellularly in the presence of quercetin, an inhibitor of DHA
uptake, suggesting that extracellular ascorbic acid concentration increased via
blocking DHA uptake by quercetin and reducing extracellular DHA by Hcy. The
effect of homocysteine on DHA reduction and uptake was confirmed with human
umbilical vein endothelial cells. The oxidation of Hcy also prevented the
decrease in DNA synthesis in human umbilical vein endothelial cells, which would
occur following exposure to Hcy.
Reduction of dehydroascorbic acid by homocysteine - ScienceDirect
https://www.sciencedirect.com/science/article/abs/pii/S0304416500001860
Hum Mol Genet. 2010 Oct 1;19(19):3721-33. doi: 10.1093/hmg/ddq286. Epub 2010 Jul
16.
Mitochondrial GLUT10 facilitates dehydroascorbic acid import
and protects cells against oxidative stress: mechanistic insight into arterial
tortuosity syndrome.
Lee YC1, Huang HY, Chang CJ, Cheng CH, Chen YT.
Author information
1
Institute of Biomedical Sciences, Academia Sinica, Taipei 11529, Taiwan,
Republic of China.
Abstract
Mutations in glucose transporter 10 (GLUT10) alter angiogenesis and cause
arterial tortuosity syndrome (ATS); however, the mechanisms by which these
mutations cause disease remain unclear. It has been reported that in most cells,
mitochondria are the major source of reactive oxygen species (ROS). Moreover,
mitochondria are known to incorporate as well as recycle vitamin C, which plays
a critical role in redox homeostasis, although the molecular mechanism(s)
underlying mitochondrial vitamin C uptake are poorly understood. We report here
that GLUT10 localizes predominantly to the mitochondria of smooth muscle cells
and insulin-stimulated adipocytes, where GLUT10 is highly expressed. We further
demonstrate that GLUT10 facilitates transport of l-dehydroascorbic acid (DHA),
the oxidized form of vitamin C, into mitochondria, and also increases cellular
uptake of DHA, which in turn protects cells against oxidative stress. This
protection is compromised when GLUT10 expression in mitochondria is inhibited.
In addition, we found that aortic smooth muscle cells from GLUT10-mutant mice
have higher ROS levels than those from wild-type mice. Our results identify the
physiological role of GLUT10 as the mitochondrial DHA transporter, and
demonstrate that GLUT10 protects cells from oxidative injury. Furthermore, our
findings provide a mechanism to explain the ascorbate in mitochondria and show
how loss-of-function GLUT10 mutations may lead to arterial abnormalities in ATS.
These results also reinforce the importance of vitamin C and ROS in degenerative
diseases.
PMID: 20639396 DOI: 10.1093/hmg/ddq286
[Indexed for MEDLINE]
Mitochondrial GLUT10 facilitates dehydroascorbic acid import and protects cells
against oxidative stress: mechanistic insight into arterial tortuos... - PubMed
- NCBI
https://www.ncbi.nlm.nih.gov/pubmed/20639396

.gif)






.jpg)