缺氧适应性策略(HIF)-乳酸-NDRG3信号通路 Hypoxia Adaptive Strategy-Lactate-NDRG3 Pathway
Hydroxylation of HIF-1: Oxygen Sensing at the Molecular Level
Gregg L. Semenza 2004
Abstract
The ability to sense and respond to changes in oxygenation represents a fundamental property of all metazoan cells. The discovery of the transcription factor HIF-1 has led to the identification of protein hydroxylation as a mechanism by which changes in Po2 are transduced to effect changes in gene expression. Multicellular life on Earth is based on the use of O2 for the efficient generation of high-energy compounds, and O2 consumption increases with the mass and metabolic activity of the organism. However, exposure to O2 must be limited due to the damaging effects of reactive oxygen species (ROS) on cellular macromolecules. Thus all of the major physiological systems of mammals participate in complex homeostatic mechanisms that are designed to maintain the O2 concentration to which each cell is exposed within a narrow range (FIGURE 1). The study of these systems has occupied physiologists for centuries. During the course of the past century, these studies have been extended to the cellular level. Finally, research over the past decade has produced dramatic insights into the molecular mechanisms underlying oxygen homeostasis during both prenatal and postnatal life.

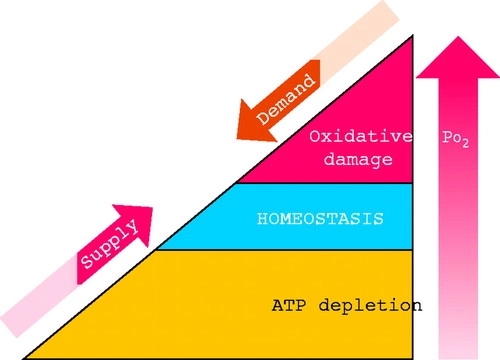
FIGURE 1.Oxygen homeostasis All of the major physiological systems participate in complex homeostatic mechanisms that regulate O2 supply and demand to maintain cellular oxygenation within a narrow range that balances the risks associated with O2 deficiency and excess.
Control of Oxygen-Regulated Gene Expression by HIF-1 Physiological responses involve changes in gene expression. The blood O2-carrying capacity is maintained by the O2-regulated production of erythropoietin (EPO), which stimulates the proliferation and survival of red blood cell progenitors. Analysis of cis-acting sequences required for increased transcription of the EPO gene in response to hypoxia led to the identification (70), biochemical purification (81), and molecular cloning (79) of hypoxia-inducible factor-1 (HIF-1). EPO is produced primarily within a rare cell type in the kidney. However, HIF-1 is expressed in all cell types and functions as a master regulator of oxygen homeostasis by playing critical roles in both embryonic development and postnatal physiology. HIF-1 has been identified in all metazoan species that have been analyzed from Caenorhabditis elegans to Homo sapiens (organ-isms whose cell numbers differ by more than 10 orders of magnitude), suggesting that the appearance of HIF-1 represented an adaptation that was essential to metazoan evolution. The expression of over 70 genes is known to be activated at the transcriptional level by HIF-1, and specific HIF-1 binding sites have been identified for many of these genes. Although the list of HIF-1 target genes is extensive (FIGURE 2), it probably underestimates the total number of genes regulated by HIF-1 by at least an order of magnitude. The battery of genes regulated by HIF-1 is different in each cell type, and, for some genes, expression can be induced or repressed by HIF-1 depending on the cell type (34).Among the critical physiological processes regulated by HIF-1 target genes are erythropoiesis, angiogenesis, and glycolysis, which are examples of systemic, local tissue, and intracellular adaptive responses to hypoxia, respectively (30).
FIGURE 2.Representative HIF-1 target genes Hypoxia inducible factor-1 (HIF-1) activates the transcription of genes encoding secreted signaling proteins, including angiogenic growth factors and survival factors, cell surface receptors, extracellular matrix proteins and modifying enzymes, transcription factors, cytoskeletal proteins, proapoptotic proteins, and glucose transporters and glycolytic enzymes. ADM, adrenomedullin; ADRA1B, α1B-adrenergic receptor; ALD, aldolase; ANGPT, angiopoietin; CITED, CREB binding protein (CBP)/p300-interacting transactivator; COL5A1, collagen V α1-subunit; CTSD, cathepsin D; CXCR, chemokine receptor; DEC, differentiated embryo chondrocyte expressed; EDN, endothelin; ENO, enolase; EPO, erythropoietin; ETS, erythroblastosis virus transforming sequence; FN, fibronectin; GLUT, glucose transporter; GPI, glucose phosphate isomerase; HK, hexokinase; KRT, keratin; LDHA, lactate dehydrogenase A; LEP, leptin; MMP, matrix metalloproteinase; NIP, BCL2/adenovirus E1B 19-kDa-interacting protein; NIX, NIP3-like; P4HA1, prolyl-4-hydroxylase α1-subunit; PFKFB3, 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase-3; PFKL, phosphofructokinase L; PGF, placental growth factor; PGK, phosphoglycerate kinase; PLAUR, urokinase-type plasminogen activator receptor; PROK, prokineticin (endocrine gland-derived VEGF); STC, stanniocalcin; TF, transferrin; TFRC, transferrin receptor; TGFA and TGFB, transforming growth factor-α and -β; TPI, triose phosphate isomerase; VEGFR, VEGF receptor; VIM, vimentin.
HIF-1 is a heterodimeric protein that is composed of HIF-1α and HIF-1β subunits. The amino-terminal half of each subunit consists of basic helix-loop-helix and Per-ARNT-Sim (PAS) domains that mediate heterodimerization and DNA binding. The carboxy-terminal half of HIF-1α contains two transactivation domains that mediate interactions with coactivators such as CREB binding protein (CBP) and p300 (30, 31, 59). Coactivators interact with both sequence-specific DNA binding proteins such as HIF-1 and with the general transcription factors associated with RNA Polymerase II (reviewed in Ref. 69). Coactivators also have histone acetyltransferase activity that is required to make the DNA embedded in chromatin accessible to the polymerase complex for transcription into RNA. The HIF-1β subunit is constitutively expressed, whereas the expression and activity of the HIF-1α subunit are precisely regulated by the cellular O2 concentration. HIF-1α accumulates instantaneously under hypoxic conditions and on reoxygenation is rapidly degraded, with a half-life of <5 min in posthypoxia tissue culture cells (28, 79). This represents an overestimate of the half-life, because it includes the time required for O2 to diffuse out of the culture medium. In an isolated, perfused, and ventilated lung preparation subjected to hypoxia and reoxygenation, the half-life of HIF-1α is <1 min (85). No protein has been shown to have a shorter half-life.
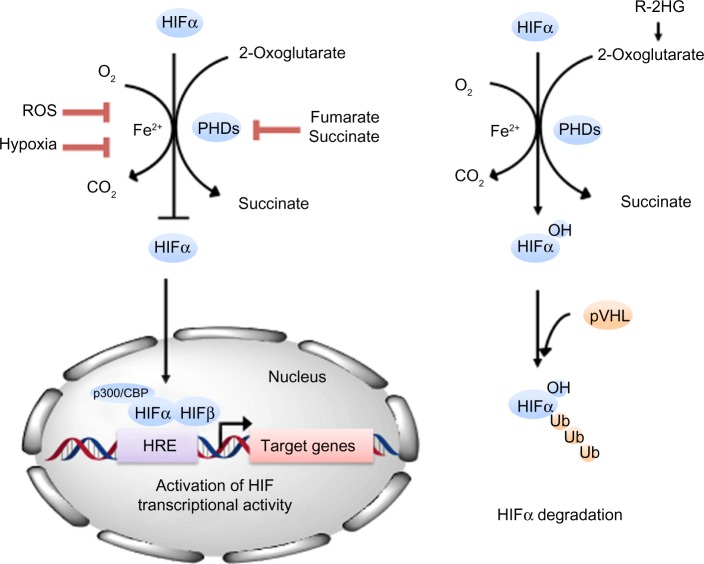
In addition to HIF-1α, a structurally and functionally related protein designated HIF-2α, which is the product of the EPAS1 gene, can also heterodimerize with HIF-1β (77). HIF-1α:HIF-1β and HIF-2α:HIF-1β heterodimers appear to have overlapping but distinct target gene specificities (22, 73). Unlike HIF-1α, HIF-2α is not expressed in all cell types, and when expressed it can be inactive as a result of cytoplasmic sequestration (56). A third protein, designated HIF-3α, has also been identified (18). Its role has not been well defined, although a splice variant, designated IPAS, has been shown to bind to HIF-1α and inhibit its activity (45, 46). Molecular Mechanisms of Oxygen Sensing The mechanism underlying the dramatic regulation of HIF-1α protein expression was a source of great debate, with several models proposed that invoked, for example, the functioning of an O2-binding hemoprotein or an ROS-generating NADPH oxidase as central to the oxygen sensing that determined HIF-1α levels (reviewed in Ref. 66). Among the observations used to support these models was the finding that HIF-1 DNA binding activity and target gene expression were induced in cells exposed to the iron chelator desferrioxamine or to cobalt chloride (80). Remarkably, HIF-1α transactivation domain function is also induced in cells exposed to hypoxia, iron chelation, or cobalt chloride (30, 31, 59), suggesting a common mechanism for regulating both HIF-1α expression and activity. The O2-dependent degradation of HIF-1α involves ubiquitination and degradation by the 26S proteasome (23, 32, 61). The von Hippel-Lindau tumor suppressor protein (VHL) is required for this process (FIGURE 3), because renal carcinoma cells lacking functional VHL constitutively express HIF-1α and HIF-1 target genes under nonhypoxic conditions (6, 49).VHL forms a complex with elongin B, elongin C, cullin 2, and RBX1 to form an E3 ubiquitin-protein ligase capable of functioning with E1 ubiquitin-activating and E2 ubiquitin-conjugating enzymes to mediate the ubiquitination of HIF-1α (33).
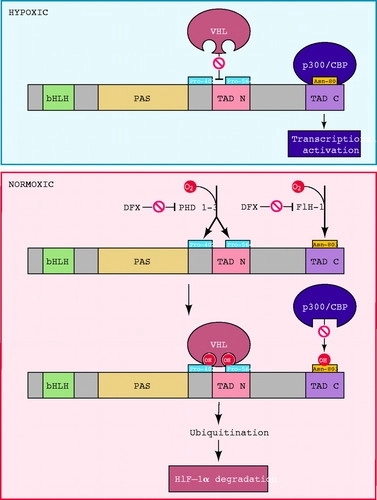
FIGURE 3.Oxygen sensing by hydroxylation of HIF-1α The amino-terminal half of HIF-1α consists of basic helix-loop-helix (bHLH) and Per-ARNT-Sim homology (PAS) domains. The carboxy-terminal half contains the transactivation domains (TAD-N and TAD-C). The HIF-1α prolyl hydroxylases (HPH)/prolyl hydroxylase domain proteins (PHD) 1–3 hydroxylate Pro-402 and Pro-564. Factor inhibiting HIF-1 (FIH-1) hydroxylates Asn-803. Proline hydroxylation is required for the interaction of HIF-1α with the von Hippel-Lindau tumor-suppressor protein (VHL), which is the recognition component of an E3 ubiquitin-protein ligase that targets HIF-1α for proteasomal degradation. Asparagine hydroxylation prevents the interaction of HIF-1α with the coactivators CBP and p300. The enzymes, which contain Fe(II) at the active site, can be inactivated by desferrioxamine (DFX) and other iron chelators. O2 appears to be a rate-limiting substrate for the hydroxylases under physiological conditions, thus providing a mechanism for the direct regulation of the stability and activity of HIF-1α as a function of the cellular O2 concentration.
A region of HIF-1α encompassing amino acid residues 400–600 is necessary and sufficient for O2-regulated ubiquitination and degradation (23, 32, 74). VHL interacts, via its β-domain, with amino acid residues 532–585 of HIF-1α (55, 75). Because the ubiquitination and degradation of other key regulatory proteins such as IκB are regulated by phosphorylation, great effort was made to identify phosphorylatable (serine, threonine, tyrosine) residues of HIF-1α that were important for regulation of protein half-life, but to no avail. Instead, Pro-564 is hydroxylated in an O2-dependent manner, and this modification is required for VHL binding (25, 27, 87). Pro-402 represents a second site of hydroxylation and VHL binding (48). Pro-402 and Pro-564 are each contained within a similar amino acid sequence (LXXLAP, where A is alanine, L is leucine, P is proline, and X is any amino acid). HIF-2α and HIF-3α expression are also regulated by prolyl hydroxylation and VHL binding (20, 49, 50).
Three prolyl hydroxylases were identified in mammalian cells and shown to use O2 as a substrate to generate 4-hydroxyproline at residue 402 and/or 564 of HIF-1α (2, 13, 24). These proteins are homologues of EGL-9, which was identified as the HIF-1α prolyl hydroxylase in C. elegans by genetic studies (13). Alternative designations for the three mammalian homologues include EGL-9 homologue (EGLN), prolyl hydroxylase domain protein (PHD), and HIF-1α prolyl hydroxylase (HPH) 1–3. The hydroxylation reaction also requires 2-oxoglutarate (α-ketoglutarate) as a substrate and generates succinate as a side product. Ascorbate is required as a cofactor. The prolyl hydroxylase catalytic site contains an Fe(II) ion that is coordinated by two histidine and one aspartate residue. Unlike heme-containing proteins, the Fe(II) in 2-oxoglutarate-dependent oxygenases can be chelated or substituted by Co(II), rendering the enzyme inactive. Most importantly, these prolyl hydroxylases have a relatively high Km for O2 that is slightly above its atmospheric concentration, such that O2 is rate limiting for enzymatic activity under physiological conditions (13, 20).As a result, changes in the cellular O2 concentration are directly transduced into changes in the rate at which HIF-1α is hydroxylated, ubiquitinated, and degraded. However, a thorough analysis of the relationship between O2 concentration and enzyme activity for each of the PHDs in living cells, and a comparison with the corresponding dose-response curve for HIF-1α expression (29), has not yet been reported. In particular, the plot of HIF-1α protein levels as a function of O2 concentration in HeLa cells yielded a sigmoidal curve suggestive of cooperativity (29), a finding that is not readily explained by the known biochemistry of the HIF-1α prolyl hydroxylases.
Remarkably, HIF-1α transactivation domain function is regulated by O2-dependent hydroxylation of Asn-803, which blocks the binding of the coactivators CBP and p300 (41). Factor inhibiting HIF-1 (FIH-1), which was identified in a yeast two-hybrid screen as a protein that interacts with and inhibits the activity of the HIF-1α transactivation domain (44), functions as the asparaginyl hydroxylase (19, 40). As in the case of the prolyl hydroxylases, FIH-1 appears to use O2 and 2-oxoglutarate and contain Fe(II) in its active site (11, 42, 51), although it has a Km for O2 that is three times lower than the prolyl hydroxylases (37). Spectroscopic analyses of a peptide from the HIF-1α transactivation domain complexed with the interacting domain of CBP or p300 revealed that Asn-803 is present within an α-helix that is buried deep within the protein interface, where it participates in multiple hydrogen-bonding interactions that are predicted to stabilize the complex (9, 12, 15). Hydroxylation of Asn-803 is predicted to disrupt these protein-protein interactions.Similarly, hydroxylation of Pro-564 has been shown to also function as a molecular switch to positively regulate the interaction of HIF-1α and VHL (21, 53). Thus hydroxylation provides a mechanism for regulating protein-protein interactions, similar to the effect of phosphorylation and other posttranslational modifications. However, what sets hydroxylation apart is that the modification occurs in an O2-dependent manner, thus establishing a direct link between cellular oxygenation and HIF-1 activity.
One remarkable aspect of the O2-sensing system described above is its plasticity. Although O2 may be the limiting substrate for hydroxylation under physiological conditions, it appears that under pathophysiological conditions iron or ascorbate may also be limiting (36). Furthermore, the expression of the PHDs varies from one cell type to another as well as in response to various physiological stimuli, including hypoxia (1, 10, 13, 52). Thus the O2dose-response curve may be shifted to the left or right under different developmental or physiological conditions. Alternative splicing of the primary RNA transcripts for two of the PHDs provides yet another mechanism for modulating prolyl hydroxylase activity (20). Finally, the transcriptional response elicited by a hypoxic stimulus also demonstrates a remarkable degree of plasticity, because the battery of target genes that is regulated by HIF-1 is unique to each cell type (34). Thus the identification of the molecular components of the O2-sensing system represents a milestone, rather than a finish line, on the course to defining the physiology of oxygen homeostasis.
Developmental and Physiological Consequences of HIF-1 Activity
The identification of HIF-1, VHL, FIH-1, and the PHDs over the past decade has delineated a pathway by which cells sense O2 and respond to changes in oxygenation with changes in gene expression, a property that is fundamental to the cells of all metazoan species. Coincident with these dramatic molecular discoveries have been equally dramatic discoveries regarding the remarkable variety of biological processes in which HIF-1 plays an important role. Analyses of mice, in which expression of HIF-1α has been lost either in all cells (germline knockout) or a single cell lineage (conditional knockout), have identified multiple aspects of development and physiology that are dependent on HIF-1 (TABLE 1). Indeed, the study of HIF-1’s role in development and physiology provides a basis for unifying these two central areas of biology. O2 delivery to cells of the developing embryo becomes limited by diffusion such that establishment of a functioning circulatory system is required for embryonic survival by embryonic day 9 (E9) in the mouse. In wild-type mouse embryos, HIF-1α expression increases dramatically between E8.5 and E9.5, whereas embryos that lack HIF-1α expression die between E9.5 and E10.5 and show cardiac malformations,vascular regression, and massive cell death (7, 26, 39, 60). Complete HIF-2α deficiency is also associated with embryonic lethality (58, 76), and because the embryos survive longer than Hif1a−/− mice, effects on multiple organ systems can be demonstrated (63).
TABLE 1. Developmental and physiological roles of HIF-1 as established by analysis of HIF-1α-null mice and cell lines Enlarge table
Whereas complete HIF-1α deficiency results in developmental defects, partial HIF-1α deficiency is sufficient to result in impaired responses to physiological stimuli. A particularly dramatic example is the loss of O2 sensing in the carotid body of Hif1a+/− mice (35). Although the carotid bodies are anatomically and histologically normal and depolarize normally in response to cyanide application, they show essentially no response to hypoxia. Thus partial HIF-1α deficiency in the carotid body results in a complete loss of the ability to sense and/or respond to changes in the arterial Po2 by stimulation of the central nervous system cardiorespiratory centers. HIF-2α is also expressed in the mouse carotid body (76), which suggests that HIF-1α and HIF-2α play distinct roles in this organ. The HIF-1 target genes that are critical for O2 sensing and/or efferent responses by the carotid body have not been identified. Remarkably, in the intact animal, other chemoreceptors are less sensitive to Hif1a gene dosage and compensate for the loss of carotid body activity in Hif1a+/− mice (35).
Another dramatic phenotype is the complete inability of Hif1a−/− myeloid cells (granulocytes and macrophages) to respond to inflammatory stimuli (8). Myeloid cells are dependent on glycolysis for ATP generation, perhaps reflecting the hypoxic microenvironment that is often associated with inflammation and infection. HIF-1α deficiency results in ATP deficiency, which impairs critical myeloid cell functions such as aggregation, motility, invasion, and bacterial killing. The role of HIF-1 in immunity is not restricted to myeloid cells, because HIF-1 also plays critical roles in B lymphocyte development (38) and T lymphocyte activation (47). The ability to create mice in which HIF-1α deficiency is restricted to a limited number of cell types (8, 62, 64) is likely to result in the identification of an increasing number of developmental and physiological processes that are regulated by HIF-1.
Medical Consequences of HIF-1 Activity The preceding sections provide a brief summary of the critical role of HIF-1 in understanding oxygen sensing, development, and physiology. HIF-1 plays an equally important role in disease pathophysiology, including ischemic cardio-vascular disease (3, 67) and cancer (68, 82), the most common causes of mortality in the US population. As a result, there is considerable interest in HIF-1 as a therapeutic target in these disorders (16, 68, 82). In the case of cardiovascular disease, increased HIF-1 activity induced as a result of HIF-1α gene therapy (34, 72, 78), small molecule inhibitors of prolyl hydroxylase activity (20, 24, 43), or inhibitors of HIF-1α-VHL interaction (83) may provide a means to stimulate neovascularization of ischemic tissue. In contrast, small-molecule inhibitors of HIF-1 activity may be useful as anticancer agents (84). However, because HIF-1 functions as a global regulator of oxygen homeostasis, it may not be a useful therapeutic target if the treatment results in unintended and undesirable side effects. An alternative approach may be to focus on the products of HIF-1 target genes. For example, erythropoietin administration may reduce ischemia-induced apoptosis in patients presenting with acute cerebral or myocardial infarction (4, 14, 54, 57). The translation of a rapidly growing body of basic science data into clinical applications looms as the most challenging and most important goal in this exciting field.
AUTHOR NOTES
gsemenza@jhmi.edu
Hydroxylation of HIF-1: Oxygen Sensing at the Molecular Level | Physiology
https://www.physiology.org/doi/full/10.1152/physiol.00001.2004
Prolyl hydroxylase domain enzymes: important regulators of cancer metabolism
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5045062/
Hypoxia (Auckl). 2014; 2: 127–142. Published online 2014 Aug

图一:说明乳酸盐是癌症的关键因素。DC,树突细胞,EC,内皮细胞;GLUT,葡萄糖转运体;IL,白介素;HAT,组蛋白乙酰基转移酶;LDH,乳酸脱氢酶;MCT,单羧酸转运体;PEP,磷酸烯醇丙酮酸;PGAM,磷酸甘油酸酯变位酶;PPP,戊糖磷酸途径;活性氧,活性氧;TAF,肿瘤相关成纤维细胞。
Lactate: A Metabolic Key Player in Cancer | Cancer Research
https://cancerres.aacrjournals.org/content/71/22/6921.long
NDRG3过表达与肝癌患者预后不良有关
NDRG3 overexpression is associated with a poor prognosis in patients with hepatocellular carcinoma
研究机构:江苏大学附属句容人民医院
缺氧诱导因子(HIFs)和N-myc下游调控基因3 (NDRG3)的表达受脯氨酸羟化酶域(PHD)酶的氧依赖性调控。
N-myc下游调控基因是一个新发现的基因家族,由NDRG1、2、3、4组成。NDRG组分通过调控靶基因转录,影响细胞增殖、凋亡、分化和发病[23,24]。因此,它们在人类肿瘤发生过程中起着至关重要的作用[25,26]。系统发育分析表明,人类NDRG1和NDRG3属于一个亚家族,而NDRG2和NDRG4属于另一个[10]亚家族。
N-myc下游调控基因3
(NDRG3)是NDRG家族的重要成员,参与细胞增殖、分化等生物学过程。本研究分析了NDRG3在肝细胞癌(HCC)中的表达,探讨了NDRG3在HCC患者中的表达与其临床病理特征的关系。我们对HCC组织进行实时定量逆转录聚合酶链反应(qRT-PCR)和免疫组化(IHC)分析,以阐明NDRG3在HCC患者中的表达特征。采用Kaplan-Meier生存曲线和Cox回归分析评价102例HCC患者的预后。结果显示,与非肿瘤组织相比,HCC组织NDRG3表达明显增高。此外,我们的分析显示NDRG3表达与肿瘤大小(P=0.048)和病理分级(P=0.001)有统计学意义。生存分析和Kaplan-Meier曲线显示,NDRG3表达是HCC患者无病生存(P=0.002)和总生存(P=0.005)的独立预后指标。这些数据表明,NDRG3的表达可能被认为是一种致癌的生物标志物和预测HCC预后的新方法。
介绍
肝细胞癌(HCC)是世界上最常见的恶性肿瘤之一。在恶性肿瘤中,HCC的患病率全球排名第五,死亡率全球排名第二。在2015年,超过20,000例HCC死亡病例发生在美国[2]。在中国,HCC新发病例和死亡病例占全球总病例(40万例)的一半以上。中国东部江苏省启东县是世界上肝癌发病率最高的地区。HCC的发生发展是一个多因素、多阶段、连续的过程,与乙型肝炎、丙型肝炎病毒感染、酒精损伤、非酒精性脂肪肝等因素相关[5,6]。近20年来,肝癌治疗如化疗、微波消融、手术切除、射频、肝移植[7]等均有进展,但肝癌的高转移和高复发率说明其总体预后仍不理想。考虑到这一点,以及预后相关因素的复杂性,必须确定预后预测因素以改进HCC的临床治疗。
N-myc下游调控基因3
(NDRG3)是NDRG家族的主要成员之一,在细胞增殖、分化等生物学功能中发挥重要作用[9,10]。NDRG3在睾丸、卵巢、前列腺、脊髓和原始胸腺中高表达,其转录产物在大脑中表达最高,其次是心脏和肾脏[11]。NDRG3存在于输精管上皮的外层,提示其可能参与了精子形成[12]的过程。近年来,NDRG3在肿瘤发生中的复杂作用引起了人们的关注。NDRG3促进前列腺癌组织的细胞生长。细胞内NDRG3的过度表达可上调CXCL1、CXCL3、CXCL5等血管生成因子,其主要作用是促进血管生长,促进肿瘤生长[13,14]。NDRG3在乙型肝炎病毒(HBV)相关的肝细胞癌中过表达,因此,它是HCC[15]的一个潜在治疗靶点。NDRG3过表达也被认为与非小细胞肺癌(non-small
cell lung cancer,
NSCLC)、前列腺癌和喉部鳞癌相关[16-18]。这些结果提示NDRG3具有促肿瘤作用;然而,最近的一份报告表明,NDRG3下调可能参与乳腺癌的发生和进展到晚期[19]。然而,目前还没有研究探讨NDRG3在HCC中的预后价值,这需要进一步的阐明。
在本研究中,我们使用实时定量逆转录聚合酶链反应(qRT-PCR)来分析NDRG3在HCC标本及其邻近非肿瘤组织中的表达。此外,我们还进行了组织芯片(TMA)和免疫组化(IHC)来检测NDRG3在HCC组织中的表达。最后,我们还评估了NDRG3表达与HCC患者临床病理特征的关系,特别是NDRG3表达与预后特征的关系。
讨论
N-myc下游调控基因是一个新发现的基因家族,由NDRG1、2、3、4组成。NDRG组分通过调控靶基因转录,影响细胞增殖、凋亡、分化和发病[23,24]。因此,它们在人类肿瘤发生过程中起着至关重要的作用[25,26]。系统发育分析表明,人类NDRG1和NDRG3属于一个亚家族,而NDRG2和NDRG4属于另一个[10]亚家族。目前,关于NDRG1在肿瘤中的表达存在不同的观点。一项研究表明,NDRG1可能通过抑制肿瘤生长或诱导细胞凋亡而成为结直肠癌(CRC)的抑癌因子。具体来说,NDRG1可以阻断march
-8诱导的受体4的降解,表达NDRG1的CRC细胞对针对死亡受体的试剂更敏感,如肿瘤坏死因子相关的凋亡诱导配体(TRAIL)[27]。NDRG1在食管鳞癌(ESCC)中的过表达也被报道与这些患者的短OS相关。据报道,NDRG1在ESCC细胞中过表达可激活Wnt信号通路,诱导上皮-间质转化(EMT),从而减少E-cadherin的表达,增加蜗牛的表达[28]。肿瘤抑制因子NDRG2依赖于抑制E3泛素连接酶Skp2活性,诱导CRC细胞分化为[29]。Hypoexpression
NDRG2也可能激活诱导EMT
NF-κB信号通路,从而大大增加的数量和大小口腔鳞状细胞癌(OSCCs)入侵的可能性以及颈部淋巴结[30]。有限的NDRG3和NDRG4研究表明,NDRG4在结直肠癌和胶质母细胞瘤中具有抑癌作用[31-33]。NDRG3在几种癌症中都有报道。据报道,与亲代或模拟空载体转染的PC-3细胞相比,外源性NDRG3在体外过表达可导致克隆数量、迁移能力和生长速度增加,在裸鼠体内过表达NDRG3可提高异种移植瘤的生长。这些结果提示NDRG3在前列腺癌的发生和发展中起关键作用。Fan等人[15]报道,NDRG3在HCC标本中表达上调,抑制NDRG3可降低肝癌细胞的恶性表型。最近的一项研究表明,NDRG3上调在NSCLC标本和细胞系中均可检测到;因此,其表达可能是NSCLC[18]的一个新的预测因子。NDRG3的研究主要集中在确定其生物学机制。据报道,通过NDRG3-Raf-ERK轴的乳酸诱导反应有助于维持肿瘤在长期缺氧条件下的进展[34-36]。有证据表明NDRG3是一种新的有用的人类癌症生物标志物;然而,尽管有报道称NDRG3在HCC标本中表达上调,但其临床病理意义及其与HCC预后的关系尚不清楚。我们需要更多的研究来确定NDRG3作为HCC的潜在治疗靶点。
我们的qRT-PCR结果显示,HCC标本的NDRG3
mRNA水平高于正常非肿瘤组织。同样,TMA和IHC分析也显示,HCC组织中的NDRG3蛋白水平显著高于非癌组织。这些结果与以往的研究结果一致[15-18],共同表明NDRG3在恶性肿瘤中表达上调。此外,NDRG3在HCC标本中的阳性表达与某些临床病理参数(如肿瘤大小和病理分级)呈正相关。Du等人[37]提出miR-31的上调可能通过抑制NDRG3的表达来减少HCC的发生,这与我们之前的研究结果一致。这些结果进一步表明NDRG3可能在HCC发生中起关键作用。
单因素和多因素分析显示,NDRG3表达与HCC患者的DFS相关,可以作为DFS的独立预测因子,而NDRG3表达和肿瘤大小被认为是影响OS的独立预后因素。此外,Kaplan-Meier分析还表明,NDRG3表达阳性的患者比NDRG3表达阴性的患者寿命更短。结果与既往研究结果一致,NDRG3在肿瘤中的过表达促进了肿瘤的发展,并与不良预后相关[16-18]。
相反,有研究表明NDRG3在某些肿瘤类型如乳腺癌中表达降低,从而表现出抑癌作用。这种差异可能是由于肿瘤起源和潜能的不同
NDRG3在不同肿瘤类型中的功能差异,如NDRGs[38-40]。
本研究存在一定的局限性。例如,我们没有检测到NDRG3在淋巴结肿瘤细胞中的表达,这可能是NDRG3参与HCC生长和转移的关键。此外,本研究以TMA标本为基础,对TNM期、HCV感染及HCC患者饮酒史了解有限且缺乏信息;因此,这些结果可能是有偏见的。我们将继续改进我们的实验设计。综上所述,本研究检测了NDRG3在HCC标本中的表达差异,并首次探讨了NDRG3表达与HCC患者临床特征的关系,特别是NDRG3的预后功能。NDRG3被认为是HCC患者新的预后标志物,可能为HCC患者提供新的治疗方法。
NDRG3 overexpression is associated with a poor prognosis in patients with
hepatocellular carcinoma | Bioscience Reports | Portland Press
https://portlandpress.com/bioscirep/article/38/6/BSR20180907/98276/NDRG3-overexpression-is-associated-with-a-poor
NDRG3和NDRG4是两个新的肿瘤相关基因
N-myc下游调控基因NDRG3和NDRG4被认为在生物学过程和发病机制中发挥重要作用。NDRG3和NDRG4的表达在许多肿瘤细胞系和肿瘤类型中被发现减少或缺失,提示它们可能发挥抑癌基因的功能。本文综述了NDRG3和NDRG4的分子结构、细胞和组织分布、生物学功能以及在肿瘤中的作用等方面的研究进展。我们试图展示它们在疾病研究中的重要性和治疗潜力。
研究机构
a.济宁医科大学,山东济宁
b 济宁医科大学附属医院,山东济宁
c 中国山东省日照市日照市人民医院
NDRG3 and NDRG4, two novel tumor-related genes - ScienceDirect
https://www.sciencedirect.com/science/article/abs/pii/S0753332213000632
乳酸引起的对缺氧的反应
A Lactate-Induced Response to Hypoxia
Medical Genomics Research Center, Korea Research Institute of Bioscience and
Biotechnology (KRIBB), Daejeon 305-806, Korea
突出了
NDRG3是PHD2/VHL通路的氧调节底物
•乳酸与NDRG3结合,增加其在缺氧状态下的水平
NDRG3激活rafe - erk信号,介导乳酸引发的缺氧反应
总结
生物体必须能够在许多体内平衡和病理环境下对低氧做出反应。通过低氧诱导因子(HIF)调节低氧反应已经得到了很好的证实,但是有证据表明,其他与HIF无关的机制也参与其中。在这里,我们报告了一种依赖于乳酸积累的缺氧反应,乳酸是一种代谢物,其产量在缺氧条件下增加。我们发现NDRG3蛋白在常氧条件下以PHD2/
vhl依赖的方式降解,但通过与在缺氧条件下积累的乳酸结合而不被破坏。稳定的NDRG3蛋白结合c-Raf介导缺氧诱导的raferk通路激活,促进血管生成和细胞生长。抑制细胞内乳酸的产生可消除ndrg3介导的缺氧反应。因此,我们的研究阐明了乳酸诱导缺氧信号传导的分子基础,为开发针对缺氧诱导疾病的治疗方法提供了依据。
介绍
氧稳态是后生动物生理学的基础。在低氧条件下,细胞依靠缺氧诱导的反应来适应和生存于恶劣的环境(Cassavaugh和Lounsbury,
2011)。缺氧反应是胚胎发育和出生后正常生理机能的重要组成部分。它们也是许多疾病的病理生理成分,包括癌症、炎症和心血管疾病。
低氧诱导因子(HIFs)通过控制一系列低氧反应基因的表达在不同的过程中发挥作用,包括新陈代谢、氧传递、pH调节、血管生成、细胞增殖和生存,从而在低氧反应中发挥核心作用(Harris,
2002, Cassavaugh和Lounsbury,
2011)。特别是,hif介导的糖酵解上调和抑制柠檬酸(TCA)循环是缺氧早期的一个关键的适应性反应(Cassavaugh和Lounsbury,
2011)。低氧诱导因子的表达和活动是由氧依赖性的羟基化严格监管的α亚基(西门,2003)。
越来越多的证据表明,缺氧有许多方面不能单独用hif介导的机制来解释。例如,抑制hif介导的通路并不总是阻止肿瘤生长;肿瘤来源于HIF-1α-deficient胚胎干细胞(ES)细胞生长优势由于低氧诱导细胞凋亡的减少和增加压力诱导增殖(Carmeliet
et al .,
1998)。许多报道表明,肿瘤血管生成是hif无关肿瘤发生的主要途径。因此,当HIF1A在ES细胞中敲除时,血管生成得以保留(Hopfl等,2002)。一些证据表明,促血管生成因子,血管内皮生长因子(VEGF)可以通过hif依赖和hif独立途径诱导(Mizukami
et al., 2004)。诱导其他pro-angiogenic等因素引发保存HIF-1α-deficient结肠癌细胞的血管生成反应(Mizukami et
al .,
2005)。此外,除HIFs外,已知有多种通路和转录因子(TFs)对缺氧作出反应,以一种与hif无关的方式诱导生物反应。在这些oxygen-regulatable
TFs
NF-κB、AP-1和CEBP激活在缺氧(康明斯和泰勒,2005)。因此,有几篇报道表明,缺氧调控的一些基因不受HIFs调控,提示其他氧调控通路的作用类似于HIF通路,由prolyl羟化酶域(PHD)酶控制(Elvidge
et al., 2006)。此外,许多蛋白激酶如PKA、PKC、PI3K、AKT、JNK、PTK2B (Pyk2)、SRC、MAPK14
(p38)和ERK1/2在缺氧条件下被激活(Seta et al.,
2002)。然而,尽管进行了所有这些研究,缺氧反应中与hif无关的分支的氧依赖调节的关键因素和机制仍然不清楚。
在本研究中,我们鉴定了一种氧调节蛋白,NDRG3
(NDRG家族成员3;NM_032013),作为PHD2/VHL系统的真实基质。NDRG3在不同的细胞类型中,在缺氧条件下均有高的诱导活性,但其mRNA的表达不受HIF水平的影响。有趣的是,糖酵解NDRG3需要绑定的最终产品为其缺氧积累乳酸,呈现其表达间接依赖于低氧诱导因子表达HIF-1α调节低氧乳酸脱氢酶的表达(LDHA)。我们发现NDRG3在乳酸诱导的缺氧信号中起关键作用,它通过介导raferk通路的激活来促进长时间缺氧时的血管生成和细胞生长。因此,NDRG3为长期缺氧反应的氧和乳酸依赖调节提供了一个关键的遗传因素。
结果
鉴定NDRG3为PHD2的底物
为了识别缺氧反应的调节因子,我们在MCF-7细胞中通过标记为PHD2的免疫沉淀和质谱联用法寻找PHD2结合蛋白。候选人中丰富的蛋白质乐队可再生产地展示微分免疫沉淀反应模式模拟和PHD2-Flag分数之间,我们选择NDRG3进行进一步的研究,因为它属于基因家族与细胞增殖、迁移、入侵以及在分化和发展(Melotte
et al ., 2010),这是与缺氧生物功能密切相关(2011年,2002岁的哈里斯Cassavaugh和劳恩斯伯里)(图S1A)。
为了详细描述NDRG3,我们在NDRG家族成员中开发了一种针对NDRG3的亲和纯化多克隆抗体(图S1B)。该抗体在PHD2-Flag免疫沉淀片段中检测到NDRG3为42-KDa条带(图1A)。我们通过免疫沉淀内源性NDRG3和缺氧培养的HeLa细胞中的PHD2-Flag来验证NDRG3-
phd2的相互作用(图1B),并直接使用重组PHD2-His和NDRG3-
gst蛋白进行下拉实验(图S1C)。因此,我们认为NDRG3是一种真正的phd2结合蛋白。
然后,我们使用PHD抑制剂desferrioxamine
(DFX)检测PHD2和NDRG3之间可能的功能关系。虽然NDRG3的基础水平表达可以忽略不计,但PHD抑制导致其在HeLa(图1C)和MCF-7细胞中的剂量依赖性积累(图S1D)。这些结果与另外两种PHD抑制剂,二甲基xaloylglycine
(DMOG)和CoCl2(图S1E)重复,表明NDRG3蛋白表达可能受到PHD介导的翻译后控制。然后,我们使用小干扰rna
(sirna),检测了不同的PHD家族成员在常氧条件下通过沉默其表达来参与NDRG3的调控。分析结果表明,对于HIF-1α,PHD2
NDRG3表达的主要监管机构的博士家庭成员(图1
d,左)。在共免疫沉淀试验中,NDRG3和PHD2之间的差异相互作用得到了证实(图S1F)。VHL的缺失,E3泛素连接酶复合物的靶向元件,也导致了NDRG3在常氧条件下的积累(图1D,右),提示NDRG3可能是PHD2/VHL介导的翻译后修饰的靶点。为了更彻底地解决这一点,我们准备了几个NDRG3蛋白的变体,它们携带单一氨基酸,在假定的PHD2-对接位点上发生变化,这是通过假定的NDRG3结构与已发表的PHD2结构之间的对接模型预测的(Chowdhury
et al., 2009)(图S1G)。共免疫沉淀实验表明,NDRG3突变体可以根据其phd2结合强度排序如下:V296D > Q97E >
R47D≈N66D,有趣的是,这似乎与它们在常氧条件下的蛋白表达水平呈负相关(图1E)。此外,NDRG3突变体对PHD2共免疫共沉淀的ha标记的VHL蛋白量更高(图1E),表明NDRG3与PHD2和VHL的相互作用是其蛋白表达的关键决定因素。接下来,在体内泛素化实验中,NDRG3过表达可增加NDRG3免疫沉淀的泛素量,而不同的短发夹rna
(shRNAs)沉默其表达可降低泛素量(图1F和S1H)。此外,MG132抑制蛋白酶体可显著增加HeLa细胞中NDRG3的检测水平(图S1I)。综上所述,NDRG3是一种与PHD2相互作用的蛋白,其表达受PHD2/
vhl介导的蛋白酶体途径负调控。
氧依赖调节NDRG3蛋白的表达
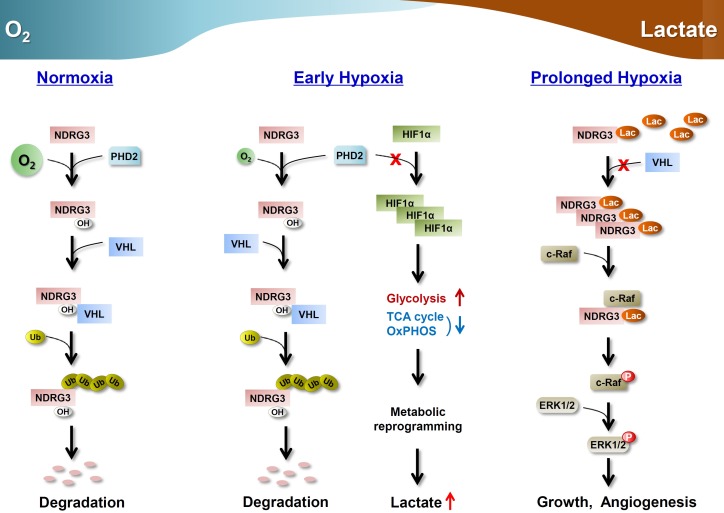
由于PHD2的活性在很大程度上依赖于O2的有效性,我们检测了NDRG3蛋白的表达是否受氧依赖的调控。NDRG3在MCF-7细胞中的积累速率与O2浓度呈负相关(图2A和S2A)。与此一致,缺氧条件下HeLa细胞NDRG3泛素化被显著抑制(图2B)。NDRG3的低氧诱导在不同组织来源的癌细胞和非转化细胞中都得到了证实(图S2B),这表明了这一现象的普遍性。然而,相比之下HIF-1α蛋白质显示一种钟形感应模式在缺氧的早期阶段,NDRG3展出一个s形的表达模式,开始当HIF-1α水平开始下降,并一直延续到后期缺氧(图2)。随着细胞复氧,NDRG3的低氧表达逐渐减少(图S2C)。这些结果强烈提示NDRG3蛋白表达受氧负调控。
接下来,我们研究了NDRG3蛋白表达的氧依赖性调控的分子基础。质谱分析表明,NDRG3在脯氨酸294处特异性羟基化,可能是PHD2修饰后的产物(图2D)。定点突变的脯氨酸294到丙氨酸(P294A)导致明显的变异蛋白的积累在常氧条件下(图2E,左)。此外,共免疫沉淀实验表明,与野生型相比,P294A突变蛋白对PHD2和VHL蛋白的结合亲和力明显降低(图2E,右),这表明脯氨酸294是PHD2介导的羟基化的关键靶位点,它决定了NDRG3蛋白在常氧条件下的稳定性。
自HIF-1α立即表达之前的NDRG3(图2 c),我们调查的可能性HIF-1α转录调节NDRG3表达式在缺氧。RT-PCR分析显示,缺氧时NDRG3
mRNA水平几乎保持不变,即使HIF蛋白达到峰值(图S2D)。这一结果表明了NDRG3转录的HIF独立性,并证实了NDRG3在缺氧条件下表达的翻译后性质。HIF不同亚基的缺失对NDRG3
mRNA水平无影响,证实了其转录的HIF独立性(图2F)。值得注意的是尽管NDRG3蛋白表达在HIF-silenced细胞缺氧显然可检测,这是相比显著降低控制HIF-1β击倒,在一个小得多的程度上,由HIF-1α击倒,暗示潜在non-transcriptional
HIF通路NDRG3蛋白质表达的影响。同时,我们可以发现HIF是另一个NDRG家族成员NDRG1低氧表达的转录激活因子(图S2E)。这些结果共同表明,缺氧时NDRG3表达的转录调控并不需要HIF活性。
NDRG3在调节缺氧反应中的作用
我们通过将NDRG3的蛋白表达谱与代表缺氧反应的五个基因本体论范畴的基因组活动谱相关联,研究了NDRG3在缺氧中的潜在功能(图S3A)。通过基因集富集分析估计基因本体论的基因组活动,根据缺氧时特定时间点Huh-7细胞转录组表达数据计算标准化差异分数(Z分数)。结果表明,NDRG3蛋白表达与“血管生成”、“抗凋亡”、“增殖(阳性)”、“运动”功能高度相关,与“糖酵解”无关(图3A)。另一方面,NDRG3在缺氧24小时时耗竭,当细胞内NDRG3蛋白表达本应达到显著水平时,导致“血管生成”、“抗凋亡”、“增殖(阳性)”、“运动”类活性发生显著变化,而“糖酵解”类活性未发生显著变化(图3B)。相比之下,“糖酵解”明显的目标HIF-1α消耗6人力资源在缺氧,当HIF-1α蛋白表达预计达到峰值水平(图S3B)。一致地,NDRG3的一个常氧稳定变体的异位表达(图1E中的N66D)导致了具有血管生成、增殖、≈生长、≈凋亡、≈迁移、糖酵解等主要功能的基因的上调(>1.5倍)(图S3C)。
然后我们对NDRG3在“血管生成”、“抗凋亡”和“增殖”中的作用进行了实验评估,这些功能通常与肿瘤的生长有关,并被NDRG3的耗竭显著靶向(图3B)。在HUVEC细胞成管实验中,NDRG3耗竭显著抑制了缺氧诱导的Huh-7细胞的血管生成活性(图S3D)。与此同时,基质凝胶塞实验表明NDRG3敲除可抑制BALB/c-nu小鼠Huh-7细胞的血管生成活性(图3C)。在分子水平上,缺氧诱导的促血管生成标记物的表达被NDRG3耗尽而被NDRG3上调(N66D)(图3D)。接下来,通过caspase-3/7和PARP裂解实验检测NDRG3的抗凋亡活性,发现NDRG3的耗竭显著促进缺氧时的细胞凋亡(图3E)。因此,缺氧诱导的抗凋亡基因的表达,特别是IAP(凋亡蛋白抑制剂)家族的成员,通过NDRG3的耗竭在Huh-7细胞中被消除(图3F)。此外,使用针对其3’-UTR的shRNA(图S1H, #5)消耗NDRG3显著抑制了轻度缺氧(3% O2)下Huh-7细胞的生长;但是这种表型被缺乏NDRG3自然3’-UTR序列的重组NDRG3(N66D)表达载体有效拯救(图3G和S3E)。此外,NDRG3基因敲除还能显著抑制BALB/c-nu小鼠Huh-7细胞的肿瘤生长(图3H和S3F)。有趣的是,NDRG3和HIFs中的任何一种同时缺失都完全抑制了肿瘤的生长,这表明NDRG3和HIFs在缺氧细胞生长中具有互补作用(图3H)。切除肿瘤的免疫荧光显微镜显示,NDRG3的缺失有效地抑制了肿瘤血管生成(IL8和CD31)和细胞增殖(Ki-67)标志物的表达,而hif缺失肿瘤的标志物表达水平与对照组相当(图S3G)。相比之下,NDRG3(N66D)的异位表达在软琼脂中高度促进了Huh-1细胞的集落形成(图S4J)以及BALB/c-nu小鼠的致瘤活性(图3I和S3H)。这些结果表明NDRG3在缺氧时促进血管生成、抗凋亡和细胞增殖方面起着关键作用。
l-乳酸触发ndrg3介导的缺氧反应
HIF-1α相比,显示在缺氧早期感应模式,迅速消失在复氧的细胞,NDRG3开始积累相对缺氧及其水平后缓慢下降后再氧化(图2
c和S2C)。NDRG3的积累和降解存在较长的滞后期,提示其低氧表达可能涉及多层调控。因此,我们探索了除低氧水平外与“长时间缺氧”相关的生化特征,发现NDRG3蛋白表达与细胞乳酸生产高度相关;缺氧时,NDRG3蛋白的表达开始于∼6小时,与乳酸盐的产生模式非常接近(图4A)。另一方面,使用LDHA抑制剂-草酸钠抑制乳酸盐的产生,可以以剂量依赖的方式特异性地抑制NDRG3蛋白的积累(图4B)。类似地,通过sirna介导的LDHA消耗(图4C)或2-脱氧葡萄糖的糖酵解中断(图S4A)来抑制乳酸盐的生成,可以抑制缺氧的NDRG3蛋白的表达。葡萄糖和/或谷氨酰胺是糖酵解和谷氨酰胺分解的输入底物,分别是导致细胞内产生乳酸的两种主要代谢途径。通过剥夺细胞内葡萄糖和/或谷氨酰胺,NDRG3蛋白的积累也随之减少,但不会影响NDRG3的转录(图4D)。然而,与葡萄糖剥夺的显著后果相比,谷氨酰胺效应似乎相对较小。相反,促进乳酸生产(通过LDHA过表达和/或丙酮酸过食;图S4B)或其胞内积聚(通过阻断MCT4的出口;图4C)增强了NDRG3蛋白的缺氧积累。这些结果表明,与HIF蛋白不同,缺氧本身并不足以导致NDRG3蛋白的积累,但还需要糖酵解产生乳酸。
然后,我们通过向细胞提供外源性乳酸来更直接地验证乳酸对NDRG3蛋白动力学的影响,这些细胞的细胞内乳酸的产生受到遗传或药理手段的影响。乳酸添加剂量依赖性Huh-1细胞恢复体内缺氧NDRG3蛋白表达,减少LDHA沉默,不影响NDRG3
mRNA水平或HIF-1α蛋白(图4
e)。当葡萄糖剥夺(图S4C)或草酸盐处理(图S4D)抑制乳酸的产生时,也得到了类似的结果。然而,以MCT1为靶点的siRNA破坏了乳酸介导的NDRG3蛋白表达恢复(图4F)。我们还观察到,在谷氨酸盐处理或葡萄糖剥夺的Huh-1细胞中,MCT1的下调也有类似的效果(图S4E和S4F)。综上所述,这些结果表明NDRG3蛋白在缺氧状态下需要乳酸积累,这说明NDRG3可能作为一种缺氧诱导的乳酸传感器,触发细胞内hif无关的生物反应。
因此,我们研究了缺氧NDRG3表达在乳酸代谢中的功能意义。在轻度缺氧条件下,用草酸酯抑制乳酸的产生导致了Huh-1细胞生长的剂量依赖性抑制(图S4G)。然而,通过体外表达NDRG3(N66D)有效地挽救了这一效应(图S4G),表明NDRG3可能在乳酸诱导的缺氧细胞生长中发挥关键作用。显然,NDRG3(N66D)不受谷氨酸盐处理的直接影响(图S4H),其表达也不受谷氨酸盐的影响(图S4I),说明NDRG3(N66D)本身确实具有拯救作用。使用Huh-1细胞的群体形成实验进一步证实了谷氨酸盐对NDRG3(N66D)细胞生长及其拯救的影响(图S4J)。然后我们检测了NDRG3在细胞生长中的作用,这些细胞的LDHA表达被RNAi去除。shRNA对LDHA的消耗抑制了Huh-1细胞在轻度缺氧条件下的生长(图S4K)以及BALB/c-nu小鼠的肿瘤生长(图4G和S4L)。NDRG3(N66D)在体内外都有效地弥补了LDHA缺陷。此外,在HUVEC细胞成管实验中,在缺氧条件下,oxamate抑制了Huh-1细胞诱导的血管生成活性(图4H)。然而,NDRG3(N66D)的异位表达恢复了这些细胞的血管生成活性,尽管有oxamate。因此,乳酸似乎是缺氧细胞生长和血管生成的关键信号,而NDRG3是乳酸诱导缺氧反应的关键中介。
乳酸调节NDRG3蛋白表达的分子机制
通过研究乳酸对NDRG3泛素化的影响,探讨了乳酸诱导NDRG3蛋白积累的分子机制。在体外,乳酸盐通过HEK293T细胞的PHD2/VHL复合物免疫沉淀催化NDRG3泛素化(图5A),表明乳酸盐可以阻断PHD2/VHL对NDRG3蛋白的修饰。乳酸显然不会影响HIF-1α蛋白表达在缺氧(图4和图S4)。因此,我们通过研究这两种分子之间的相互作用来研究乳酸盐直接调节NDRG3的可能性。利用gst标记的重组NDRG3蛋白和[14C]标记的l-乳酸盐进行的体外结合实验表明,NDRG3在物理上和直接结合乳酸盐(图5B和S5B)。为了进一步验证NDRG3-乳酸相互作用,我们通过对接模拟(未显示)预测了NDRG3假定的乳酸结合域。预测的乳酸结合域定点突变表明,其部分氨基酸残基的突变会损害突变蛋白的低氧积累(图S5A)。其中一个glycine-138突变为色氨酸(图S5A中的N3(G138W))的突变体在缺氧条件下几乎没有积累,但在MG132存在的情况下积累,这表明它可能失去了逃避PHD2/
vhl介导的蛋白酶体降解所必需的乳酸结合能力。事实上,我们观察到重组N3(G138W)-GST蛋白在体外结合实验中有严重的乳酸结合能力受损(图5C和S5B)。这些结果表明,乳酸结合抑制蛋白酶体降解的NDRG3阻断其修饰PHD2/VHL。
此外,NDRG3-乳酸复合物一旦形成,似乎仍然对PHD2/
vhl介导的修饰具有相当的抗性,因为缺氧积累的NDRG3蛋白在常氧条件下的新鲜培养基中培养后维持了一段时间(图S2C)。相比之下,HIF-1α迅速消失在复氧,展示精美的氧依赖性的翻译后的监管。
我们通过对HEK293T细胞中表达表位标记的NDRG3、PHD2和VHL的蛋白结合分析,进一步探讨了乳酸诱导NDRG3蛋白动态变化的机制。NDRG3与PHD2在缺氧早期(6小时)或缺氧晚期(24小时)的结合与常氧状态下无显著差异,提示低氧或高乳酸水平均不影响NDRG3-PHD2的相互作用(图5D)。相比之下,NDRG3与VHL在缺氧24小时时结合明显减少,而在缺氧6小时时则维持在常氧水平,这说明高乳酸水平而非低氧水平可能影响NDRG3-VHL的相互作用。然后,我们使用在PHD2
(P294A)或乳酸结合(G138W)中具有prolyl羟基化缺陷的NDRG3变体来验证这些观察结果。野生型或变异NDRG3物种在常氧和缺氧(24小时)条件下的phd2结合能力均无显著差异(图5E)。另一方面,野生型NDRG3的vhl结合能力在缺氧条件下较常氧条件下明显降低,而P294A和G138W的vhl结合能力在缺氧条件下几乎没有变化。值得注意的是,P294A与VHL的相互作用可以忽略不计,而G138W-VHL的相互作用则在不考虑氧含量的情况下得到了强烈的维持。野生型NDRG3的泛素化在缺氧条件下显著降低,而P294A和G138W在常氧和缺氧条件下的泛素化均可忽略且强度较强(图5F)。相反,oxamate处理特异性地增强了野生型NDRG3与VHL的低氧相互作用以及其泛素化(图5G和S5C)。葡萄糖剥夺抑制低氧乳酸的产生也导致NDRG3-VHL相互作用的增强(图S5D)。在缺氧和常氧条件下,在草酸处理的细胞中添加外源性乳酸可特异性地抑制NDRG3-VHL相互作用(图S5E)。因此,我们得出结论,NDRG3-PHD2相互作用不受细胞氧或乳酸水平的影响,而NDRG3-VHL相互作用受乳酸水平的显著抑制,而不受低氧水平的影响。
综上所述,缺氧时产生的过量乳酸直接与NDRG3结合,通过阻断NDRG3-
vhl相互作用,抑制其泛素化和蛋白酶体降解。然而,NDRG3泛素化在高乳酸条件下的失败是否由于抑制了phd2介导的NDRG3的羟基化,这是VHL结合所必需的,有待进一步研究。
缺氧时NDRG3激活raferk信号
为了了解NDRG3在缺氧条件下的分子机制,我们通过使用稳定表达NDRG3或GFP
shRNA的PLC/PRF/5细胞进行磷阵列分析,寻找可能的NDRG3调控激酶(图S6A)。NDRG3耗竭选择性地抑制缺氧诱导的ERK1/2磷酸化(图6A和S6A)。然后我们检测了ERK1/2上游的激酶是否可以被NDRG3调控,发现缺氧诱导的c-Raf
(at Ser338)和B-RAF1 (at
Ser445)的磷酸化被SK-Hep-1细胞中的NDRG3耗竭所消除(图6B)。这些结果提示NDRG3可能在激活raferk信号通路中发挥重要作用。因此,我们研究了操纵NDRG3表达对c-Raf磷酸化的影响,发现异位表达的c-Raf在常氧条件下显著磷酸化,同时伴有ERK1/2磷酸化(图6C)。然而,siRNA缺失了NDRG3的碱基表达,就取消了这种反应。另一方面,NDRG3(N66D)的异位表达高度诱导了c-Raf和ERK1/2的磷酸化(图6C)。此外,缺氧诱导内源性c-Raf和ERK1/2的磷酸化被NDRG3的3’-UTR-targeting
shRNA抑制,重组NDRG3(N66D)表达载体可以挽救(图6D)。体外双向下拉实验表明,NDRG3可以与c-Raf发生直接的物理作用(图S6B)。
在缺氧条件下,异位表达的c-Raf免疫沉淀内源性NDRG3蛋白具有特异性(图S6C)。此外,在体外激酶实验中,HEK293T细胞沉淀的含NDRG3(N66D)的复合免疫沉淀介导了重组c-Raf的磷酸化(图6E)。这些结果表明NDRG3直接参与了c-Raf的磷酸化。
图缩略图figs6
图s6与NDRG3相关的蛋白激酶信号分析,与图6相关
显示完整的标题
查看大图查看器下载高清图片下载(PPT)
图缩略图gr6
图6NDRG3是缺氧诱导的raferk1 /2激活所需要的
显示完整的标题
查看大图查看器下载高清图片下载(PPT)
然后我们研究了ndrg3介导的c-Raf-ERK1/2磷酸化的生物学意义。通过对内源性蛋白的免疫沉淀分析,我们观察到,在缺氧过程中,c-Raf-
ndrg3复合物的数量增加,同时c-Raf和ERK1/2的磷酸化水平也随之增加(图6F)。这一结果提示ndrg3介导的c-Raf-ERK1/2磷酸化可能在缺氧反应调节中发挥作用。通过消融LDHA抑制乳酸的产生,有效抑制了缺氧诱导的c-Raf和ERK1/2的磷酸化和NDRG3蛋白的表达(图6G)。通过外源提供的乳酸盐,可以抑制sildhai介导的c-Raf和ERK1/2磷酸化,但这种抑制被沉默MCT1的表达所阻断。此外,葡萄糖剥夺对糖酵解的破坏可以有效抑制c-Raf和ERK1/2的低氧磷酸化以及NDRG3的表达,NDRG3可以挽救这一过程(N66D)(图6H)。相比之下,缺乏谷氨酰胺的影响微乎其微。这些结果表明,缺氧诱导的c-Raf和ERK1/2的磷酸化依赖于乳酸的产生,主要来自糖酵解,而NDRG3是乳酸诱导的raferk通路激活的重要中介。
乳酸诱导的缺氧细胞生长和血管生成依赖于ndrg3介导的ERK1/2活性
最后,我们研究了ndrg3介导的rafe -
erk通路激活与乳酸盐引起的缺氧反应的生物学相关性。外源提供的乳酸能显著弥补轻度缺氧时LDHA沉默引起的Huh-1细胞生长缺陷(图7A)。然而,通过NDRG3的耗竭或ERK信号的药理阻断,乳酸介导的抢救被取消。同样,外源性乳酸可恢复低氧诱导的LDHA-knockdown
Huh-1细胞在成管实验中的血管生成能力,而NDRG3耗竭或ERK抑制又可消除这种能力(图7B)。与此同时,血管生成标记基因的低氧表达,被LDHA敲除后,由外源性乳酸恢复,但被NDRG3耗竭或ERK抑制后再次中断(图S7A)。然后我们检测了ndrg3介导的raferk通路激活与体内肿瘤生长的相关性。Western
blot分析体内肿瘤发生分析中,针对LDHA和/或NDRG3表达而设计的Huh-1细胞形成的肿瘤(图4G和S4L)表明,与模拟对照组(图7C和S7B)相比,表达NDRG3(N66D)的肿瘤中c-Raf和ERK的磷酸化明显上调。我们一致观察到NDRG3(N66D)表达肿瘤中血管生成标记基因的主要表达。这些结果以及图4和图6中的结果表明,在缺氧条件下,乳酸盐在促进细胞生长和血管生成方面起着重要作用,这取决于ndrg3介导的c-Raf-ERK1/2通路的激活。
然后,我们通过对人肝癌(HCC)的免疫组织化学分析,检测了NDRG3表达和ERK1/2活性的临床相关性。NDRG3在正常肝脏中几乎不表达,而在细胞质和质膜中的HCC组织中可检测到中高表达(图7D)。在103例使用NDRG3和phospho-ERK1/2抗体检测的HCC病例中,25例(24.3%)NDRG3蛋白表达阳性,与ERK1/2激活显著相关(图7D)。总之,这些结果表明NDRG3的异常表达与体内肿瘤的发展以及ERK通路的病理激活密切相关。
讨论
乳酸一直被认为是糖酵解和谷氨酰胺分解的终端产物,直到最近才作为替代能源和肿瘤血管生成的诱导物出现(Doherty和Cleveland,
2013)。LDHA表达的下调或其活性的抑制抑制了体内外肿瘤细胞的生长(Fantin et al., 2006, Le et al.,
2010)。然而,乳酸诱导的生物反应的关键因素和机制仍然未知。在这项研究中,我们证明了ndrg3介导的乳酸信号的存在及其在缺氧反应中的作用。在缺氧、低氧和乳酸水平升高时,NDRG3蛋白的表达被高度诱导,从而激活了rafe
- erk通路,促进血管生成和缺氧细胞的生长。因此,NDRG3作为一个乳酸传感器,以缺氧依赖的方式触发下游激酶信号,NDRG3- rafe -
erk轴为乳酸诱导的缺氧反应提供了遗传基础。
我们发现NDRG3的表达与HIFs无关,在蛋白水平上由乳酸盐决定。乳酸积累在缺氧的后阶段,促进糖酵解的upregulation和LDHA表达在缺氧的早期HIF-1α扮演着关键的角色作为代谢适应的一部分(Cassavaugh和朗伯里,2011)。因此,乳酸信号和随后的生物反应似乎功能耦合HIF-1α-induced代谢重编程,采用NDRG3关键链接。在这方面,建议部分缺氧反应,特别是发生在缺氧的后阶段,迄今为止已归因于HIF-1α可能,事实上,NDRG3-mediated乳酸直接控制下的信号。基因集富集的结果分析功能NDRG3和缺氧期间HIF-1α支持这种可能性(图3和S3)。因此,我们的研究表明,HIF-1α和NDRG3可能形成一种氧依赖性的监管链缺氧反应,大致分为两个时间阶段(图7
e);在早期阶段,积累HIF-1αO2含量低信号,然后调节早期基因表达所必需的适应性反应包括代谢重编程,在后期阶段,调节乳酸生产信号NDRG3的积累,而随后激活Raf-ERK通路诱导反应所需应对长期缺氧。
缺氧信号的乳酸- ndrg3 - rafe -
erk轴提示,缺氧乳酸的产生可能是正常生理的一个组成部分,在长时间缺氧条件下促进血管生成和细胞生长。显而易见,这种功能之间的耦合HIF-1α-induced乳酸代谢重编程和NDRG3-mediated信号确保细胞面临长期缺氧缺氧环境中实现最大可能的增长。这可以通过,首先生成生物合成的积木和能量通过HIF-1α-mediated
upregulation糖酵解,随后,通过提供线索细胞生长和血管生成通过NDRG3-mediated
c-Raf-ERK信号。因此,ndrg3介导的乳酸信号可能为局部组织中的细胞从缺氧中恢复提供一个自给自足的机制,而不需要额外的细胞外信号,例如在发育期间。此外,NDRG3介导的信号转导为细胞逃避长时间缺氧提供了额外的生物安全层,因为NDRG3蛋白一旦被乳酸结合稳定下来,即使在细胞再氧化时也保持相当稳定。
越来越多的证据表明,乳酸可能在癌症进展中发挥积极作用,因为它作为一种氧化代谢物,介导了癌症细胞对代谢的固有作用,并在肿瘤微环境中对几种细胞类型的非癌症细胞自主作用(Doherty和Cleveland,
2013)。我们的结果表明,糖酵解是乳酸产生的主要来源,负责缺氧诱导NDRG3蛋白的表达和raferk的激活。癌细胞对糖酵解的依赖程度经常增加,因此,乳酸-
ndrg3 - raf -
erk轴及其在血管生成和缺氧细胞生长中的作用的发现,可能为糖酵解表型对癌症的生长优势提供了重要的解释。在这方面,乳酸可能被认为是一种单一代谢产物,作为一种替代燃料,一种调节肿瘤微环境的制剂,一种信号分子。
许多缺氧反应的特征也被病变细胞利用(Cassavaugh和Lounsbury, 2011)。缺氧的存在与癌症患者预后差、治疗效果差有关(Jubb et al.,
2010, Semenza,
2004),因此缺氧已成为癌症治疗的重要靶点。尽管低氧诱导因子在这方面的主要目标,提出了担心的简单抑制HIF可能不足以防止低氧诱导疾病的进展,因为许多研究表明,补偿,HIF-independent通路可以诱导抑制单因素时(Mizukami
et al ., 2005年,Mizukami et al ., 2007年,Carmeliet et al ., 1998年,Rapisarda et al
., 2009;参见介绍以获得支持示例)。这些观察结果表明,最成功的抗缺氧策略可能需要抑制hif独立通路和hif依赖通路的药物组合(Mizukami et
al., 2007, Fong,
2008)。尽管HIF-1α的功能耦合的可能性,NDRG3似乎在缺氧的反应截然不同的功能调节的基因集富集的转录组数据分析NDRG3
HIF1A-depleted细胞在缺氧。因此,这些观察结果,以及本研究中显示的NDRG3在缺氧反应中的作用,表明HIF和NDRG3的组合靶向可能在癌症治疗中非常有效。当NDRG3与HIFs联合使用时,肿瘤生长被抑制,这支持了该策略的可行性(图3H)。
综上所述,NDRG3为缺氧信号的缺氧依赖调节提供了重要的遗传证据。NDRG3在缺氧状态下的调控和功能表明,PHD2/VHL系统可以以氧依赖的方式控制hif依赖和非hif依赖的缺氧反应。因此,lactate-NDRG3-Raf-ERK信号通路可能提供延长机械的线索的理解障碍引起的突变VHL(成血管细胞瘤,肾细胞癌、嗜铬细胞瘤等)(Maher
et al ., 2011)或PHD2(家族erythrocytosis-3)(珀西et al .,
2006)以及hypoxia-related生理和病理生理反应(Cassavaugh和朗伯里,2011)。
A Lactate-Induced Response to Hypoxia: Cell
https://www.cell.com/cell/fulltext/S0092-8674(15)00264-0#articleInformation
NDRG3 lowers the metastatic potential in prostate cancer as a feedback
controller of hypoxia-inducible factors | Experimental & Molecular Medicine
https://www.nature.com/articles/s12276-018-0089-y
乳酸盐:代谢在癌症中起关键作用
Lactate: A Metabolic Key Player in Cancer | Cancer Research
Franziska Hirschhaeuser, Ulrike G.A. Sattler, and Wolfgang Mueller-Klieser
Authors' Affiliation: Institute of Physiology and Pathophysiology, University
Medical Center of the Johannes Gutenberg University Mainz, Mainz, Germany
增加葡萄糖的吸收和积累乳酸,即使在正常的条件下(如需氧糖酵解或Warburg效应)是癌细胞的一个共同特征。这一现象清楚地表明乳酸盐不是肿瘤缺氧的替代物。肿瘤乳酸盐可以预测转移和患者的总体生存,这是由几项不同实体的研究显示的。肿瘤的转移是由肿瘤相关的成纤维细胞分泌乳酸诱导的透明质酸来促进的,这为迁移创造了有利的环境。乳酸本身已经被发现可以诱导细胞和细胞簇的迁移。此外,耐辐射性与乳酸浓度呈正相关,提示乳酸具有抗氧化能力。肿瘤代谢物与免疫细胞相互作用的结果表明,乳酸对免疫逃逸有贡献。此外,乳酸盐在伤口愈合、慢性炎症和癌症的发展之间架起了桥梁。肿瘤细胞通过分泌乳酸诱导的VEGF,为增殖提供充足的氧气和营养,从而形成新的血管。总之,在实体瘤中乳酸盐的积累是恶性肿瘤发展的关键和早期事件。乳酸盐的测定应该进入进一步的临床试验,以确认其在癌症生物学中的相关性。癌症Res;71
(22);6921 - 5。AACR©2011。
介绍
Warburg在20世纪20年代的开创性研究和他发表的研究成果(1)之后,在肿瘤糖酵解和乳酸代谢领域进行了大量的研究。多年来,这些研究主要集中在分离的肿瘤细胞和肿瘤细胞系中碳水化合物代谢的生化调控。与此同时,肿瘤的放射治疗也在不断的推进,效率也越来越高,但似乎存在一个主要的限制:供氧受限,供氧与耗氧不平衡,导致许多肿瘤缺氧。因此,对缺氧的研究,包括其对癌症患者的原因和后果以及对治疗操作的敏感性,多年来主导了对肿瘤代谢和微环境的研究。与肿瘤缺氧相比,肿瘤糖酵解和乳酸生物学多年来很少受到科学关注。然而,有关糖酵解相关基因在全球70%的人类癌症中过表达的发现(2),以及使用18f
-氟脱氧葡萄糖的正电子发射断层摄影术(positron emission
tomography)利用癌细胞对葡萄糖的摄取增加来进行肿瘤诊断的发现,使这个话题再次复兴。如本文所述,这可能导致临床环境中癌症诊断和治疗随访的改善。我们认为乳酸盐在肿瘤代谢中起关键作用的观点如图1所示。

图1
说明乳酸盐是癌症的关键因素。DC,树突细胞,EC,内皮细胞;GLUT,葡萄糖转运体;IL,白介素;HAT,组蛋白乙酰基转移酶;LDH,乳酸脱氢酶;MCT,单羧酸转运体;PEP,磷酸烯醇丙酮酸;PGAM,磷酸甘油酸酯变位酶;PPP,戊糖磷酸途径;活性氧,活性氧;TAF,肿瘤相关成纤维细胞。
肿瘤发生中的代谢转换
正常细胞的恶性转化导致大多数实体瘤在正常缺氧条件下葡萄糖摄取和乳酸形成增加。普遍接受的是,这种现象是细胞呼吸缺陷的结果,致癌的改变,和过度的糖酵解酶和代谢产物转运蛋白(3)。许多癌基因和肿瘤抑制基因参与代谢从氧化磷酸化(OXPHOS)对肿瘤细胞糖酵解的改变,如myc、NF-κB在一种蛋白激酶/蛋白激酶B,表皮生长因子(EGF)、胰岛素样生长因子I,phosphoinositol
3激酶(PI3K)、mTOR
Kirsten鼠肉瘤病毒致癌基因相同器官(喀斯特),活化蛋白激酶(AMPK)和低氧诱导因子1α(HIF-1α;参考文献。3和4).大多数致癌基因已被证明可刺激编码介导糖酵解和谷氨酰胺分解的蛋白的基因。转录因子是细胞适应的关键成分HIF-1α,通常通过prolyl羟化酶和冯Hippel-Lindau复合物,由细胞氧气浓度控制。注意,HIF-1α通过糖酵解的产物,乳酸和丙酮酸,甚至在常氧条件下稳定,导致这个主转录因子HIF-1α的积累(5)。因此,符合华宝(1)观察,HIF-1调节基因的表达会导致肿瘤中独立于氧气的一个增强的糖酵解通量的增加。因此,实体瘤中乳酸积累的现象并不仅仅是缺氧的替代(6)。Yaromina和同事(7)表明,在微观区域水平上,高乳酸浓度与缺氧无关。(缺氧与葡萄糖代谢的相关性在其他文献中也有论述,但不是本文的主要主题。)HIF-1的作用靶点包括膜转运蛋白,如葡萄糖转运蛋白1
(GLUT-1)和一元羧酸转运蛋白4 (MCT-4),这两种蛋白既能保证足够的葡萄糖进入细胞,又能保证积累的乳酸分泌出细胞。此外,由于乳酸脱氢酶A
(LDH-A)表达增强,NAD+生成,允许继续糖酵解和ATP的产生。丙酮酸脱氢酶激酶1的HIF
-1依赖性激活以及由此导致的丙酮酸脱氢酶复合物的失活导致了氧化磷酸化通量的降低。尽管高转化率的丙酮酸转化为乳酸的糖酵解途径,一些丙酮酸还有待三羧酸(TCA)周期中用于生物能量学和生物合成的目的(4)。三羧酸循环和磷酸戊糖途径(PPP)可以保持肿瘤细胞增殖的高前体池最大化,同时损伤周围正常组织或整个有机体。此外,PPP产生NADPH作为抗氧化反应的中介,保护细胞免受氧化损伤(3)。除了糖酵解,谷氨酰胺分解是另一个主要的能量生产来源,似乎有助于提高肿瘤细胞的乳酸积累。谷氨酰胺分解还能促进增殖细胞中大分子的合成(4)。另一种乳酸来源是肿瘤特异性的丙酮酸激酶(PK)
M2,它能将磷酸烯醇丙酮酸(PEP)转化为丙酮酸。然而,PEP也能作为一个磷捐赠者对磷酸甘油酸酯变位酶1
(PGAM1),形成的丙酮酸(8)独立于PKM2的活动。总的来说,这似乎是其他糖酵解途径关键酶转向这些酶的胚胎型压型支持所需的大规模增殖(9)的一个共同特征。
最近的数据表明,糖酵解的解除可能与癌细胞基因调控的表观遗传机制有关。特别是Buchakjian和Kornbluth(10)描述了丙酮酸对组蛋白乙酰酶(HAT)和组蛋白去乙酰酶(HDAC)的影响,导致糖酵解酶和转运蛋白的转录增加。因此,糖酵解的代谢物可能参与了表观遗传反馈回路,而这一回路目前仍知之甚少,需要在这一领域进行进一步的研究。
最近的数据表明,糖酵解的代谢转变可能是肿瘤发生的早期和基础事件。Schafer及其同事(11)的研究结果表明,Her2/neu+导管乳腺癌细胞糖酵解的上调可通过阻止EGF受体的下调从而维持PI3K通路的激活,为其提供生存优势。Ong和他的同事(12)发现,与非恶性粘膜相比,癌前肠息肉以及腺癌患者的葡萄糖摄入大量增加。一些肿瘤调节分子的变换,如NF-κB、参与炎症的协调。这与早期认为癌症是“愈合过度的伤口”的观点有关。此外,大多数组织伤口愈合过程的基因也是癌症生长和进展的重要的正调控或负调控因子(13)。乳酸本身作为一种内在的炎症介质,导致T细胞和巨噬细胞产生更多的白细胞介素(IL)-17A,从而促进肿瘤微环境中的慢性炎症(14)。
乳酸对免疫逃逸的贡献
肿瘤发生的一个主要原因是免疫系统无法充分清除异常细胞。最近的研究已经详细阐述了肿瘤细胞的几种逃逸机制,包括抑制分子的上调、免疫抑制细胞因子的产生和共刺激分子的下调(15)。除了这些机制外,肿瘤代谢也在很大程度上参与了免疫逃逸。近年来,研究发现胞外乳酸可抑制单核细胞向树突状细胞(DC)的分化,并抑制树突状细胞(16)和细胞毒性T细胞(17)释放的细胞因子,而后者是抗肿瘤反应的关键。免疫分析肿瘤显示大量的肿瘤浸润淋巴细胞,这表明了缺少免疫反应并不主要是调低的MHC-1与肿瘤相关抗原调节的识别的失效,但相反,而是功能适应性免疫系统的损伤。
激活的T细胞本身以糖酵解为主要能量来源(17)。当肿瘤细胞向细胞外空间释放大量乳酸时,免疫细胞无法清除自身的乳酸,因为细胞乳酸的分泌依赖于细胞内外浓度的比值。最终,白细胞可能被乳酸盐窒息。由于细胞乳酸通过MCTs的分泌伴随着H+的运输,细胞外pH值的降低导致细胞毒性T细胞功能的降低(17,18)。相反,调节T细胞(Treg)似乎不受乳酸和酸性微环境的影响,因为它们有不同的能量代谢依赖于脂肪酸氧化(19)。这可以解释为什么实体瘤的整体免疫细胞浸润率既不能预测疾病的结果,也不能预测患者的生存。
乳酸对细胞迁移的不同影响
在经典的Boyden
chamber实验中,外源性乳酸的加入导致了多种癌细胞株随浓度的随机迁移增加(20)。这适用于与体内实体肿瘤相关的乳酸水平。,0-40更易/
L)。此外,乳酸介导的肿瘤细胞运动增强不仅表现在单细胞运动中,而且还表现在通过延时显微摄像技术强制的大量迁移中(20)。尽管乳酸诱导的信号蛋白水平变化及其激活状态,如β1-integrins已经有记载、分子机制参与乳酸对细胞活性的影响还不清楚。最近的数据支持TGF-β2信号通路是一个乳酸效应相关癌细胞迁移的调节者(21)。
Goetze和同事(20)采用与癌细胞相同的实验设置,发现外源性乳酸始终抑制单核细胞的迁移。这一发现伴随一个浓度依赖细胞因子如il -
6或TNF-α释放的减少,。需要指出的是,在整个实验过程中,pH值都被控制在7.2以下。
Boyden
chamber实验表明,乳酸通过内皮细胞(EC)刺激VEGF的生成,导致增强的迁移,并独立于O2条件下产生乳酸诱导的血管生成(22)。最近,Vegran和他的同事们(23)在这个杂志上发表了一篇文章,他们表示,乳酸吸收在ECs
MCT-1激发NF-κB活动和IL-8的表达。利用小鼠异种移植模型,他们发现肿瘤细胞通过MCT-4释放乳酸足以刺激il -8依赖性血管生成和肿瘤生长。
添加到培养的成纤维细胞中的乳酸会增加其透明质酸的产生,并导致CD44的表达升高,CD44是一种跨膜糖蛋白,是细胞表面主要的透明质酸受体。癌周围的基质增加了肿瘤相关成纤维细胞(TAF)产生的透明质酸,提供了一个促进癌细胞生长和运动的环境(24,25)。
乳酸积累在原发性肿瘤中的临床意义
在2000年,我们实验室的数据发表在本刊上,显示在长达9年的随访期间,原发性宫颈癌患者乳酸积累与患者生存率呈负相关(26)。据我们所知,这是第一个合理的证明,在临床环境下,肿瘤乳酸代谢与癌症的侵袭性密切相关。利用天然特异性LDH酶作为乳酸传感器,获得肿瘤组织中代谢物浓度的数据。这种酶在生化上与荧光素酶有关,其释放的生物荧光强度与代谢物浓度成正比。使用适当的标准,一个人可以校准光信号在单位组织浓度,如微摩尔每克组织(μmol
/
g)。在冰冻组织中诱导生物发光反应,使得根据组织的组织学结构记录光信号成为可能。本质上,诱导代谢生物发光成像(imBI)允许定量和结构相关的检测组织中的代谢物在体外的微观水平上的分辨率。由于这些独特的特性,imBI在动物和人类中的应用为基础和转化性癌症研究提供了新的信息,如下所述。
在发表上述乳酸在初级宫颈癌及其相关病人的生存的数据后不久,一个头颈部鳞状癌患者的独立研究还表明,治疗前乳酸含量与接受化疗和放射治疗后总体与无病生存率呈负相关(25、27)。此外,所有研究对象的肿瘤乳酸含量与远处转移的发生率呈显著正相关(25)。在这些研究中发现,相同分期和分级的肿瘤内的乳酸盐浓度差异很大。在分析的3个实体中,与其他代谢物(如ATP和葡萄糖)相比,活的肿瘤区域的乳酸浓度显示出非常大的肿瘤内和肿瘤间的变化。然而,瘤内乳酸盐的变化远远小于瘤间差异。利用imBI技术获得肿瘤内乳酸积累的临床相关性。目前,还没有一种非侵入性技术可以同时提供这两种优势。而且,目前还不清楚哪些种类的乳酸盐。可以通过各种侵入性和非侵入性方法检测。然而,最近的一些非侵入性研究集中在乳酸的定量及其潜在的临床意义。在与我们相似的研究中,Saraswathy和他的同事(28)发现,质子磁共振成像测量的高乳酸盐强度与多形性胶质母细胞瘤患者的低存活率有关。Park和他的同事(29)发现移植的人胶质母细胞瘤的13C乳酸水平明显高于正常的大鼠大脑。其他研究使用了13C光谱学或高分辨率的幻角纺丝技术(30)。
在靶向代谢治疗前和治疗中应用乳酸定量成像技术,可以提供有关靶点特异性、肿瘤反应和临床结果的有价值的信息。这可能是目前临床研究和实践环境中乳酸测量最重要的作用。
乳酸对辐射抵抗的贡献
来自一个研究组(包括我们的实验室)的实验肿瘤的结果,包括1000多个人类HNSCC的异种移植,表明乳酸盐浓度与辐射抵抗正相关(31)。这种相关性可能是由于,至少部分,在以前的研究中揭示的乳酸的抗氧化性能(32)。抗肿瘤治疗,如电离辐射和一些化疗药物,诱导氧化应激的目标细胞。活性氧(ROS)的过量产生会导致DNA和RNA损伤、脂质过氧化和基因组不稳定性。活性氧是固定辐射诱导的DNA损伤所必需的;因此,抗氧化剂(如乳酸盐)的积累可能诱导或增强对辐射的抵抗,并可能导致化学抵抗(33)。由于动物在接受化疗或放疗后乳酸水平下降(34),因此监测人体肿瘤中乳酸代谢产物可能有助于预测治疗反应。
总之,通过糖酵解来改变抗氧化代谢产物的水平,从而改变ROS的数量,可能会导致更好的治疗反应。
结论与未来展望
肿瘤细胞进行代谢转换,产生促进细胞生长和分裂的中间体。这似乎是一个非常早期的(如果不是最初的)致癌事件,至少在目前观察到的大量病例中如此。以我们目前的知识为基础,现在就去控制糖酵解在肿瘤发生中的作用得出确切的结论还为时过早。然而,越来越多的数据支持将这一假设作为未来肿瘤代谢基础研究的演技假设。试图阐明炎症事件、代谢异常、基因改变和功能性恶性肿瘤的序列中的“鸡和蛋问题”是实验性癌症研究中一个令人兴奋的挑战。
虽然一些遗传、生化和病理生理机制已被确定为导致高乳酸性肿瘤高度恶性的原因,但为什么看似相同的肿瘤可能在其组织中乳酸含量上表现出极端差异仍是一个谜。这无疑是该领域未来研究的又一挑战。
尽管缺少这些知识,关于肿瘤代谢的转化研究已经转移到将癌症研究的一些基本方面转移到临床环境中。Tennant和他的同事(35)编写了大量基于操纵肿瘤代谢的临床肿瘤学试验,直至III期。鼓励该领域的所有研究人员为利用肿瘤代谢诊断和治疗癌症的共同努力做出贡献。
Lactate: A Metabolic Key Player in Cancer | Cancer Research
https://cancerres.aacrjournals.org/content/71/22/6921.long
加洛黄素是一种新型的乳酸脱氢酶抑制剂,通过影响不同的信号通路,诱导不同糖酵解态度的人乳腺癌细胞死亡
Galloflavin, a new lactate dehydrogenase inhibitor, induces the death of human
breast cancer cells with different glycolytic attitude by affecting distinct
signaling pathways
摘要
最近发现的一种乳酸脱氢酶抑制剂,通过阻止糖酵解和ATP的产生来阻止癌细胞的增殖。本实验的目的是研究该化合物对乳腺癌细胞系的影响,这些细胞系可产生该肿瘤的不同病理亚型:MCF-7(高分化型)、MDA-MB-231(侵袭性三阴性肿瘤)和MCF-Tam(具有获得性三苯氧胺抗性的MCF-7亚型)。
我们观察到这些细胞系的能量代谢有显著差异。与MCF-7细胞相比,MDA-MB-231和MCF-Tam细胞均表现出较高的LDH水平和葡萄糖摄取水平,并表现出较低的耗氧量。尽管存在这些差异,但GF也有类似的生长抑制作用。这一结果可以通过在MDA-MB-231和MCF-Tam细胞中发现一种结构性激活的应激反应来解释,这些细胞复制了预后不良的肿瘤形态。进一步证明,不同的信号通路参与了GF的抗增殖作用。MCF-7细胞中我们观察到ERα-mediated信号所需的调节细胞生存。相反,MCF-Tam和MDA-MB-231细胞的生长抑制似乎是由氧化应激条件造成的。细胞死亡的普遍机制是诱导凋亡。
由于具有三重阴性和/或化学抗性表型的乳腺癌形式的临床相关性,我们的结果显示GF甚至对积极生长的细胞也有类似的作用,这鼓励进一步的研究来验证这种化合物在改善乳腺癌化疗中的潜力。
Galloflavin, a new lactate dehydrogenase inhibitor, induces the death of
human breast cancer cells with different glycolytic attitude by affecting
distinct signaling pathways
Abstract
Galloflavin (GF), a recently identified lactate dehydrogenase inhibitor, hinders
the proliferation of cancer cells by blocking glycolysis and ATP production. The
aim of the present experiments was to study the effect of this compound on
breast cancer cell lines reproducing different pathological subtypes of this
tumor: MCF-7 (the well differentiated form), MDA-MB-231 (the aggressive triple
negative tumor) and MCF-Tam (a sub-line of MCF-7 with acquired tamoxifen
resistance).
We observed marked differences in the energetic metabolism of these cell lines.
Compared to MCF-7 cells, both MDA-MB-231 and MCF-Tam cells exhibited higher LDH
levels and glucose uptake and showed lower capacity of oxygen consumption. In
spite of these differences, GF exerted similar growth inhibitory effects. This
result was explained by the finding of a constitutively activated stress
response in MDA-MB-231 and MCF-Tam cells, which reproduce the poor prognosis
tumor forms. As a further proof, different signaling pathways were found to be
involved in the antiproliferative action of GF. In MCF-7 cells we observed a
down regulation of the ERα-mediated signaling needed for cell survival. On the
contrary, in MCF-Tam and MDA-MB-231 cells growth inhibition appeared to be
contributed by an oxidative stress condition. The prevalent mechanism of cell
death was found to be apoptosis induction.
Because of the clinical relevance of breast cancer forms having the triple
negative and/or chemoresistant phenotype, our results showing comparable effects
of GF even on aggressively growing cells encourage further studies to verify the
potential of this compound in improving the chemotherapy of breast cancer.
Galloflavin, a new lactate dehydrogenase inhibitor, induces the death of
human breast cancer cells with different glycolytic attitude by affecting
distinct signaling pathways - ScienceDirect
https://www.sciencedirect.com/science/article/abs/pii/S0928098712003235
没食子酸的抗癌作用及其分子机制的研究展望
癌症是全球第二大死亡原因。人们对新型抗癌药物的需求一直很大,科学家们探索各种天然和人造化合物来克服这一问题。没食子酸(GA)是一种酚酸,存在于许多古代医药中。它具有抗炎、抗氧化、抗病毒和抗菌的特性。本文综述了赤霉素及其衍生物的抗癌活性。报道了赤霉素对多种癌细胞株的体内外实验结果。先前的研究表明,GA的抗癌活性是通过不同的机制(如诱导细胞凋亡相关代的活性氧(ROS),调节凋亡和抗凋亡蛋白,抑制和促进致癌基因,抑制基质金属蛋白酶(MMPs)和细胞周期阻滞取决于类型的癌症研究。综上所述,GA及其衍生物可作为单独治疗癌症的有效药物,也可与其他抗癌药物联合使用,提高化疗效率。然而,仍然需要更多的动物模型和人体临床试验来推广并将GA及其衍生物推向商业市场。
allic acid: prospects and molecular mechanisms of its anticancer activity Cancer is the second leading cause of death worldwide. There is always a huge demand for novel anticancer drugs and scientists explore various natural and artificial compounds to overcome this. Gallic acid (GA) is one of the phenolic acids found in many dietary substances and herbs used in ancient medicine. It possesses antiinflammatory, antioxidant, antiviral and antibacterial properties. The present review summarizes the anticancer activity of GA and its derivatives. Various in vitro and in vivo experiments of GA against a variety of cancer cell lines were reported. The previous studies show that the anticancer activity of GA is related to the induction of apoptosis through different mechanisms like generation of reactive oxygen species (ROS), regulation of apoptotic and anti-apoptotic proteins, suppression and promotion of oncogenes, inhibition of matrix metalloproteinases (MMPs) and cell cycle arrest depending upon the type of cancer investigated. Conclusively, GA and its derivatives may be considered as a potent drug for cancer treatment alone as well as in combination with other anticancer drugs to increase the efficiency of chemotherapy. However, there is still a need for more experimentation in knock-out animal models and human clinical trials to promote and place GA and its derivatives on the commercial market.
The Rate of Oxygen Utilization by Cells
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3147247/
Targeting tumor perfusion and oxygenation to improve the outcome of anticancer therapy1
Radiotherapy and chemotherapy are widespread clinical modalities for cancer treatment. Among other biological influences, hypoxia is a main factor limiting the efficacy of radiotherapy, primarily because oxygen is involved in the stabilization of the DNA damage caused by ionizing radiations. Radiobiological hypoxia is found in regions of rodent and human tumors with a tissue oxygenation level below 10 mmHg at which tumor cells become increasingly resistant to radiation damage. Since hypoxic tumor cells remain clonogenic, their resistance to the treatment strongly influences the therapeutic outcome of radiotherapy. There is therefore an urgent need to identify adjuvant treatment modalities aimed to increase tumor pO2 at the time of radiotherapy. Since tumor hypoxia fundamentally results from an imbalance between oxygen delivery by poorly efficient blood vessels and oxygen consumption by tumor cells with high metabolic activities, two promising approaches are those targeting vascular reactivity and tumor cell respiration.This review summarizes the current knowledge about the development and use of tumor-selective vasodilators, inhibitors of tumor cell respiration, and drugs and treatments combining both activities in the context of tumor sensitization to X-ray radiotherapy. Tumor-selective vasodilation may also be used to improve the delivery of circulating anticancer agents to tumors. Imaging tumor perfusion and oxygenation is of importance not only for the development and validation of such combination treatments, but also to determine which patients could benefit from the therapy. Numerous techniques have been developed in the preclinical setting. Hence, this review also briefly describes both magnetic resonance and non-magnetic resonance in vivo methods and compares them in terms of sensitivity, quantitative or semi-quantitative properties, temporal, and spatial resolutions, as well as translational aspects.
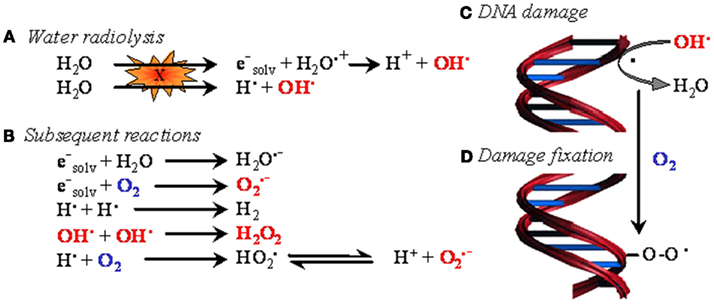
Figure 1. The oxygen enhancement effect in radiotherapy. In biological tissues, irradiation primarily induces water ionization and destabilization, leading to the formation of reactive radical species (A). These species then react with neighboring molecules to yield reactive oxygen species (ROS) (B), among which the hydroxyl radical is believed to be the most cytotoxic. When generated in the proximity of DNA, hydroxyl radicals and, to a lesser extent, other less energetic species attack DNA (C). The resulting formation of a DNA radical is readily reversible. However, in the presence of oxygen, DNA damage can be stabilized through oxidation of DNA radicals, eventually leading to the formation of DNA peroxides (D). In oncology, oxygen-dependent DNA damage fixation is known as the “oxygen enhancing effect” of radiotherapy.
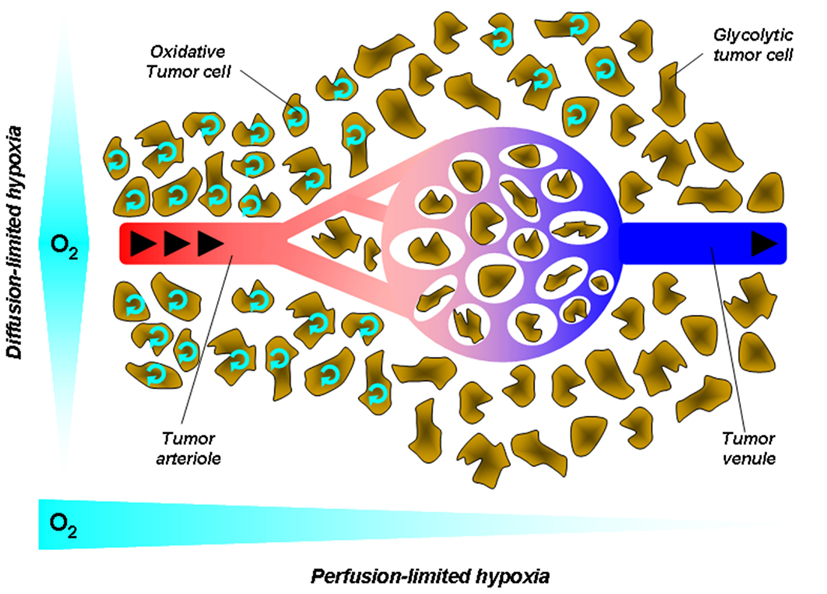
Figure 2. Tumor hypoxia. Hypoxia in tumors results from a mismatch between the oxygen supply by poorly efficient blood vessels and oxygen consumption by metabolically overactive tumor cells. This simplified cartoon depicts two main forms of hypoxia. Diffusion-limited hypoxia refers to a gradient of oxygen deprivation from the nearest perfused blood vessels toward tumor cells at increasing distances from this vessel. It originates from high-rate of oxygen extraction though layers of cells within a loosened vascular network. Perfusion-limited hypoxia refers to oxygen deprivation along the vascular tree from the tumor margin toward the tumor core. Poor oxygen delivery has many causes in tumors, including high-rate of oxygen extraction at the tumor margin, decreased red blood cell deformability, and stacking, increased blood viscosity due to water extraction, vascular disorganization, and angiogenesis. Arrows represent the blood flow.
Hypoxia, a partial pressure of oxygen (pO2) below physiological needs, is a limiting factor affecting the efficiency of radiotherapy. Indeed, the reaction of reactive oxygen species (ROS, produced by water radiolysis) with DNA is readily reversible unless oxygen stabilizes the DNA lesion. While normal tissue oxygenation is around 40 mmHg, both rodent and human tumors possess regions of tissue oxygenation below 10 mmHg, at which tumor cells become increasingly resistant to radiation damage (radiobiological hypoxia; Gray et al., 1953). Because of this so-called “oxygen enhancement effect” (Figure 1), the radiation dose required to achieve the same biologic effect is about three times higher in the absence of oxygen than in the presence of normal levels of oxygen (Gray et al., 1953; Horsman and van der Kogel, 2009). Hypoxic tumor cells, which are therefore more resistant to radiotherapy than well oxygenated ones, remain clonogenic, and contribute to the therapeutic outcome of fractionated radiotherapy (Rojas et al., 1992).
Tumor hypoxia occurs in two ways: chronic hypoxia (or diffusion-limited hypoxia), and acute hypoxia (or perfusion-limited or fluctuating hypoxia; Figure 2). Chronic hypoxia has classically been thought to result from long diffusion distances between tumor vessels as the consequence of the more rapid expansion of tumor cells than that of the supporting vasculature (Vaupel et al., 1989). It is now well established that steep longitudinal gradients of pO2 along the vascular tree, as opposed to radial diffusion of oxygen, can largely contribute to deficiencies in tumor oxygen supply (Dewhirst et al., 1999).
Hyperthermia: Combining Provascular and Oxygen Consumption Effects in a Single Treatment Hyperthermia is a potent adjuvant therapy with radiotherapy and chemotherapy, and the perfect illustration of a strategy combining transient, local vasodilatation with the inhibition of tumor cell respiration. The heat treatment consists of elevating the temperature of tumors to a supra-physiological range of 40–45°C at which tumor reoxygenation occurs with limited skin toxicity. Hyperthermia induces a graded response in tissues characterized by decreased oxygen consumption at temperatures ≥40°C, vasodilatation between 41 and 41.5°C, and vascular damage above 42°C. Although direct tumor cell killing was demonstrated in vitro at higher temperatures, long-term tumor control has never been demonstrated using hyperthermia as the sole treatment modality. Vasodilation only modestly contributes to tumor reoxygenation at the low thermal doses. Increased pO2 rather primarily results from changes in oxygen consumption in the target cells, and at least two different processes have been identified to contribute to this response. It is now well demonstrated that an important target of heat is proteins among which enzymes of the respiratory chain are more sensitive to heat inactivation/denaturation than glycolytic enzymes (Lepock et al., 1987; Kelleher et al., 1995). But the inhibition of mitochondrial respiration by heat lasts longer than the turnover time of respiratory enzymes, suggesting the existence of an additional mechanism. Dewhirst in collaboration with our team (Moon et al., 2010) recently demonstrated that mild hyperthermia activates the transcription factor hypoxia-inducible factor 1 (HIF-1) through an hypoxia-independent mechanism involving the sequential activation of Extracellular signal-Regulated Kinases (ERK) by heat shock, ERK-induced upregulation of the expression of the Nox1 subunit of NAD(P)H oxidase, increased ROS production by NAD(P)H oxidase, ROS-induced HIF-1α protein stabilization, and, ultimately, HIF-1 activation. HIF-1 target genes include most glycolytic enzymes and transporters as well as major pro-angiogenic molecules such as VEGF. Among these genes, we showed that pyruvate dehydrogenase kinase 1 (PDK1) largely mediates the inhibition of mitochondrial respiration by heat in tumor cells through inhibiting pyruvate dehydrogenase (PDH), i.e., the enzyme coupling glycolysis to the tricarboxylic acid (TCA) cycle (Moon et al., 2010). Furthermore, consistent with the increase in VEGF expression that we also observed in heat-treated tumors, we documented an increased vascular density in perfused tumor areas where oxygen is extracted from the blood. Tumor reoxygenation by mild hyperthermia is thus a multifaceted process involving the combination of decreased O2consumption by tumor cells and increased O2 delivery by blood vessels. This and the fact that reoxygenation occurs at thermal doses lower than those inducing vascular damage justifies the use of mild hyperthermia as a combination treatment notably with radiotherapy. Although several clinical trials have confirmed that combining heat and radiotherapy is indeed associated with better patient treatment outcome (Brizel et al., 1996; Jones et al., 2003, 2005; Vujaskovic et al., 2003) the future clinical development of hyperthermia strongly relies on designing tools allowing for homogeneous thermal dose distribution and improving imaging techniques able to correlate thermal maps of tumors to the clinical outcome of patients (Dewhirst et al., 2010).
Frontiers | Targeting Tumor Perfusion and Oxygenation to Improve the Outcome
of Anticancer Therapy1 | Pharmacology
https://www.frontiersin.org/articles/10.3389/fphar.2012.00094/full
Front. Pharmacol., 21 May 2012 | https://doi.org/10.3389/fphar.2012.00094
J Cancer 2016; 7(7):817-822. doi:10.7150/jca.14274
Warburg Effect - a Consequence or the Cause of Carcinogenesis?
Slobodan Devic
Department of Radiation Oncology, SMBD Jewish General Hospital, McGill
University, Montréal, Québec, Canada.
abstract
Ever since its discovery (1924) the Warburg effect (aerobic glycolysis) remains an unresolved puzzle: why the aggressive cancer cells “prefer” to use the energetically highly inefficient method of burning the glucose at the cellular level? While in the course of the last 90 years several hypotheses have been suggested, to this date there is no clear explanation of this rather unusual effect. Even though it is commonly assumed that Warburg effect is a consequence of carcinogenesis, yet another hypothesis could be brought up that the cellular switch to aerobic glycolysis may represent the very point in time when a normal cell becomes cancerous. Furthermore, this switch may happen at the point where the fate of pyruvic acid is determined, caused by the inadequate supply of enzymes that promote citric as opposed to lactic acid cycle. Currently, few clinical observations, like low cancer incidence in Type 1 diabetes mellitus and increased cancer incidence in people on high carbohydrate diets might be called upon to support such hypothesis.
Economy of cellular energy balance
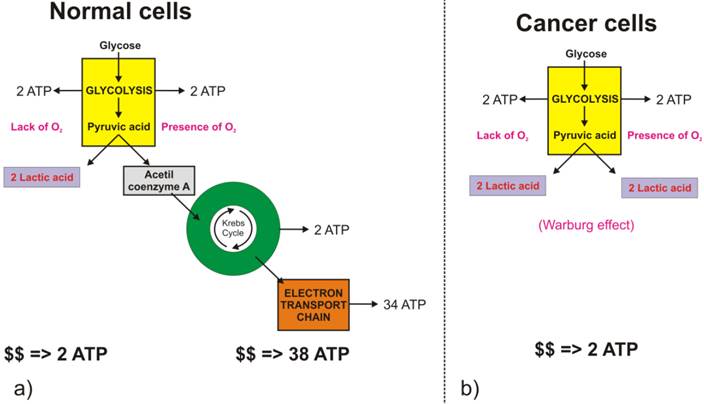
The full oxidation of one glucose molecule (oxidative phosphorylation) within a cell in the presence of oxygen produces 38 molecules of adenosine-three-phosphate (ATP), which in turn represents the essential cellular fuel (Figure 1.a). The first step (Glycolysis, occurring in cytoplasm) of glucose cellular respiration produces only 2 ATP molecules and ends up with production of two molecules of Pyruvic acid. If a given cell has access to oxygen, the Pyruvic acid will be converted to Acetyl-coenzyme A, which enters the Krebs cycle (citric acid cycle, occurring within mitochondria) followed by the electron transport chain process (occurring on the inner mitochondrial membrane) that creates most of the ATP molecules. It is the very last step within electron transport chain that needs oxygen to collect the terminal electron from the last cytochrome (cyt a3) and become a nascent O- to pick up 2 H+ and create one of the byproducts of aerobic cellular respiration - a water molecule. On the other hand, if the cells reside under hypoxic conditions, the pyruvic acid is not converted into acetyl-coenzyme A, but into a lactic acid - the process termed anaerobic cellular respiration (lactic acid cycle). In the latter case, the net energy balance is only two ATP molecules making the anaerobic glucose metabolism energetically highly inefficient process.
While the lack of oxygen at the cellular level can occur at times of excessive physical activity (resulting in subsequent muscle pain), it also represents a hallmark of highly invasive and fast-growing cancers [[1]]. As the cancer cells multiply, their fast multiplication rate outgrows the angiogenesis so much so that while the glucose access to fast-growing cells could be sufficient the lack of blood vessels disrupts the level of oxygen needed for the full glucose oxidation. Such a microscopic picture defines one of the fundamental properties of the cancer microenvironment that forces malignant cells to metabolize glucose through the lactic acid cycle. Looking at chemical equations between aerobic and anaerobic glucose metabolism (Figure 1) one may conclude that cancer cells (as well as healthy ones) under hypoxic condition would need 19 times higher uptake of glucose to maintain the same metabolic level as well-oxygenated cells. This would, in turn, mean that the appropriate quantitative analysis of FDG-based PET images could help in pinpointing hypoxic segments (by abundance only [[2]]) of the tumor by solely looking into very high uptake values, which would eventually be some 20 times greater than in well-oxygenated cancer cells. However, the isolation of the hypoxic target volumes is far from being that simple.
Warburg effect, or aerobic glycolysis - hallmark of invasive cancers Apart from the fact that acute hypoxia in tumors develop as soon as one moves few hundred microns from the blood vessels, yet another important fact prevents FDG being an ideal hypoxia marker - the Warburg effect. Recently, interest in tumor metabolism has been revived partly as a result of the widespread clinical application of PET using FDG. FDG-based PET imaging has confirmed that most primary and metastatic cancers show a significant increase in the glucose uptake when compared to normal tissues.
Warburg effect, or aerobic glycolysis - hallmark of invasive cancers Apart from the fact that acute hypoxia in tumors develop as soon as one moves few hundred microns from the blood vessels, yet another important fact prevents FDG being an ideal hypoxia marker - the Warburg effect. Recently, interest in tumor metabolism has been revived partly as a result of the widespread clinical application of PET using FDG. FDG-based PET imaging has confirmed that most primary and metastatic cancers show a significant increase in the glucose uptake when compared to normal tissues.
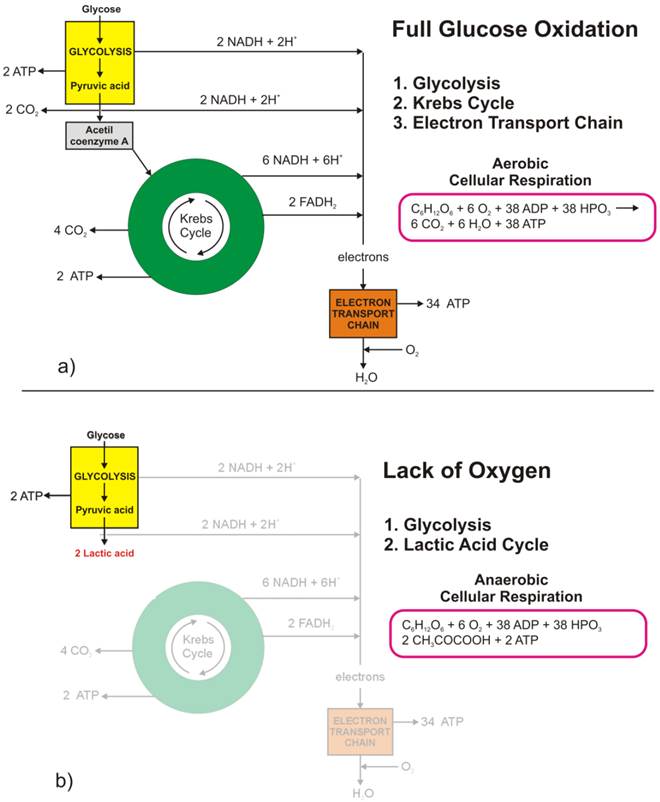
Figure 1 Glucose metabolism at the cellular level: a) full glucose oxidative phosphorylation; b) anaerobic glycolysis (lactic acid cycle). (Click on the image to enlarge.)

Figure 2 Difference in glucose metabolic pathways as a function of oxygen abundance in a) normal cells, and b) cancer cells. (Click on the image to enlarge.) Glycolysis involves the conversion of glucose to pyruvate and then to lactic acid, the waste product. In non-cancerous cells, mitochondria oxidize pyruvate to carbon dioxide and water in the presence of oxygen (Figure 2.a), and the glycolytic reaction is inhibited (Pasteur Effect [[3]]). Conversion of glucose to lactic acid, even in the presence of oxygen is known as aerobic glycolysis (Figure 2.b) or the Warburg effect [[4], [5]]. In one of his seminal papers [[6]], Warburg suggests that carcinogenesis is a two-step process. Cancer cells originate from normal cells by firstly encountering irreversible respiration injury. The second phase of cancer formation represents a long struggle for existence by the injured cells to maintain their structure, in which a part of the cells die from lack of energy while another part succeeds in replacing the irretrievably lost respiration energy by fermentation energy (from lactic acid cycle). Warburg's initial hypothesis that cancer results from impaired mitochondrial metabolism has been shown to be incorrect, but the observation of augmented glycolysis in tumors, even in the presence of oxygen, has been continually proven [[7]]. While cancer cells do carry oxidative phosphorylation, the majority of glucose molecules taken by cancer cells (66%) are metabolized through fermentation [[8]], a process that is ten times faster than full glucose oxidation.
Contemporary explanation of the Warburg effect
In addition to being energetically highly inefficient process glycolysis (either anaerobic or aerobic), with its metabolic products (such as hydrogen ions), cause constant acidification of the extracellular space, which might result in increased local toxicity [[9], [10]]. Nevertheless, despite these drawbacks, cancer cells consistently progress towards the wasteful and potentially toxic glycolytic phenotype. Gatenby and Gillies [[11]] proposed that the consistent expression of up-regulated glycolysis is not accidental but represents a solution to the environmental growth constraints during tumor development. They suggest that increased glycolysis is an essential component of the malignant phenotype and, therefore, a hallmark of invasive cancers. Transport enzymes of the Glut and hexokinase families are up-regulated in tumor cells expressing the glycolytic phenotype, and the level of Glut-1 glucose transporter expression has been shown to correlate with [18F] FDG uptake in non-small cell lung cancer, for example [[12]]. Gatenby and Gillies [11] describe the concept of carcinogenesis as a process that occurs by cellular evolution implying that common characteristics of malignant phenotypes, such as upregulation of glycolysis, are the result of active selection processes. They further argue that upregulation of glycolysis is likely to be an adjustment to hypoxia developing as pre-malignant tissue grows gradually further away from their blood supply. Also, an augmented acid production from glycolysis upregulation leads to microenvironmental acidosis and requires further adjustments through somatic evolution to phenotypes resistant to acid-induced toxicity. Finally, they conclude that cell populations that emerge from this evolutionary sequence have a compelling growth advantage, as they alter their environment through increased glycolysis in a way that is toxic to other phenotypes, but harmless to themselves.
To further support the attempt by Gatenby and Gillies [11] in explaining the cause of Warburg effect in aggressive tumors as a response to harsh environmental conditions, one might assume that cancer cells “know” a priori they will encounter severe conditions in the future. Consequently, they “decide” to switch their glucose metabolism to highly inefficient but the only possible (and highly toxic) metabolic pathway. To make their explanation more sounded, Gatenby and Gillies [11] speculate that: “… intuitively, it would seem that the Darwinian forces prevailing during the somatic evolution of invasive cancers would select against a metabolic phenotype that is more than an order of magnitude less efficient than its competitors and that is environmentally poisonous. In other words, the accepted tenet of “survival of the fittest” would seem to generally favour populations with more efficient and sophisticated substrate metabolism.” Consequently, they suggest that glycolytic phenotype in cancers is directly governed by the evolutive mechanisms, over the relatively short time frame during which tumors develop.
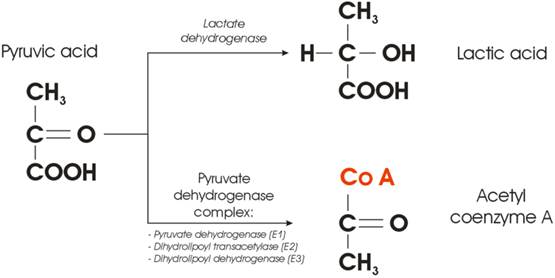
Figure 3 Enzyme-mediated cross-section between lactic and citric acid cycle.
A new hypothesis Figure 3 illustrates the very point within a cellular glucose metabolism where the fate of the Pyruvic acid is decided. In the presence of oxygen, the Pyruvic acid will be converted into acetyl group and attached to coenzyme A, a process mediated by the so-called Pyruvate Dehydrogenase Complex (PDC). The complex consists of three enzymes: pyruvate dehydrogenase, dihydrolipoyl transacetylase, and dihydrolipoyl dehydrogenase. On the other hand, conversion of pyruvic acid to lactic acid requires one enzyme only - lactate dehydrogenase (LDHA). From the cellular kinetics point of view, one may ask a simple question: what is the probability that something goes wrong with either of the two possible metabolic pathways depicted in Figure 3? Synthesis of three enzymes, needed to provide conversion of pyruvic acid to acetyl coenzyme A, even in the presence of oxygen will be (notably three times) more prone to the errors than a transcription of only one (competing) enzyme. It was reported that many human cancers have higher LDHA levels than normal tissues [[13]], but the correlation between oncogenes and glycolysis was poorly understood. The question could be then raised whether the Warburg effect is only a consequence, or could it be at the root of the very cause of carcinogenesis? A new hypothesis could be staged that the switch from aerobic cellular respiration to aerobic glycolysis leads to carcinogenesis, and the cell begins to develop the cancerous phenotype [[14], [15]] at the point where the fate of Pyruvic acid is decided. Such a switch could be governed by the lack of complete PDC (at least one of the enzymes is missing).
While at this very moment the question whether Warburg effect is a hen, or an egg remains at the level of pure hypothesis, several clinical observations might be called upon to support such proposition indirectly. While there is no apparent relation between Type 1 diabetes mellitus patients and incidence of cancer to this date [[16]], several publications argue that there could be a lower cancer rate in patients with insulin-dependent diabetes. In 2003, Zendehdel et al. [[17]] published results on cancer incidence in patients with Type 1 (insulin-dependent) diabetes mellitus on a cohort of 29 187 patients, followed over a period of 30 years, during which they observed 355 incidences of cancer. Such a low frequency (1% over 30 years, or 0.04% per year) appears negligible when compared to 1.66 million cases of new cancer cases per year in the US (0.52% per year [[18]]). Pladys et al. [[19]] reported on the lower incidence of cancer death mortality in diabetic patients (6.7%, both Type 1 and 2) when compared to non-diabetic patients (13.4%) using a cohort of 39 811 patients with the end-stage renal disease. It might be argued that cells in diabetic patients (generally deprived of normal glucose uptake due to lacking insulin) become “trained” (to use rhetoric by Blagosklonny [[20]]) by the microenvironment and well “prepared” as soon as glucose becomes available. Once the glucose is phosphorylated by hexokinase and enters the glucose oxidation process, the cell is “prepared” not to waste the opportunity and gets the maximum out of the relatively “scars glucose supplies.” One could further argue that diabetic patient cells are making sure that the synthesis of the PDC is up and running flawlessly, to avoid wasteful pathway of cellular glucose metabolism.
On the other hand, despite a relatively small amount of data published it appears that the incidence of cancer is also correlated with the increased intake of carbohydrates [[21], [22], [23]]. One may argue that normal cells, exposed to increased supply of glucose would quickly switch towards the energetically inefficient pathway (lactic acid cycle) of burning glucose even in the presence of oxygen (Warburg effect) since the source of energy (glucose-ATP) are virtually inexhaustible. In addition, the ATP production via fermentation is much faster (as mentioned above), albeit highly ineficient, when compared to full oxydation. It is also of note that contrary to type 1 (insulin-dependent), patients with type 2 diabetes mellitus have higher probability for cancer incidence [[24]]. Yet another detail deserves attention: type 1 diabetes is commonly regarded as a juvenile-onset diabetes as it often begins in childhood while the type 2 diabetes was considered an adult-onset diabetes. However, type 2 diabetes is becoming increasingly common in children [[25]] who are more obese or overweight that could be correlated with carbohydrates rich diets. Finally, the possible triggering of carcinogenesis by aerobic glycolysis, accompanied by increased glucose uptake, can be further supported by studies demonstrating increased glucose uptake observed to coincide with the transition from premalignant lesions to invasive cancer [[26], [27]].
Conclusion
Unlike Warburg's initial hypothesis that cancer cells metabolize glucose through aerobic glycolysis due to impaired mitochondrial function a new hypothesis was presented that the normal cell becomes cancerous at the point when it switches its glucose metabolism from oxidative phosphorylation to aerobic glycolysis. The new hypothesis that Warburg effect corresponds to the very beginning of carcinogenesis might be supported by the eventual failure in synthesis of the PDC. Such failure could be mediated by one (or multiple) of the well-known carcinogenic factors in synergy with an excessive supply of glucose in carbohydrates rich diets. The new hypothesis revolves around a point within cellular glucose oxidation at which the fate of pyruvic acid is decided. Several observations have been presented to support such hypothesis: lower incidence of cancer in insulin-dependent type 1 diabetes mellitus patients; increased cancer incidence in societies consuming high quantities of carbohydrates; and the increased probability of synthesis failure of the pyruvate dehydrogenase complex consisting of three enzymes when compared to the synthesis of a single lactate dehydrogenase enzyme. Contemporary agreement in explaining why tumor cells opt for aerobic glycolysis (Warburg effect) that is far less efficient than oxidative phosphorylation at producing ATP, is that it represents evolutionary adaptation to harsh microenvironmental conditions by using the carbon chains (from the lactic acid) as building blocks for synthesis of biomolecules (nucleic acids, proteins, and lipids), which are essential for cell proliferation. However, even the acceptance of the aerobic glycolysis being a more adequate glucose metabolism pathway for cancer cells, the question of a hen or an egg remains: is the Warburg effect just a consequence, or could it be the very cause of carcinogenesis (Figure 4)?
Warburg Effect - a Consequence or the Cause of Carcinogenesis?
http://jcancer.org/v07p0817.htm
代谢及其在癌症进化和治疗中的后遗症
Metabolism and Its Sequelae in Cancer Evolution and Therapy
Gillies, Robert J. PhD; Gatenby, Robert A. MD
罗伯特·j·吉利斯博士;罗伯特·盖茨比医学博士
癌症是通过一系列可以被描述为“躯体进化”的事件来发展的。“达尔文进化论的一个核心前提是,环境给了我们选择最适合特定微环境的物种的压力。此外,进化速率与(1)环境选择的强度和(2)所选种群的表型方差成正比。值得注意的是,在癌症的发展从致癌作用到当地入侵转移,选择性格局不断变化,并且在整个过程中,增加了细胞的选择,改变了代谢表型:暗示这些表型传授过程中选择优势环境的选择。一种最常见的表型是需氧糖酵解,即在有足够氧气存在的情况下葡萄糖的持续发酵。这种所谓的“Warburg效应”的机制已经得到了很好的研究,有多种模型可以解释这种现象是如何在分子水平上发生的。在此,我们提出可以通过评估产生这种表型的环境背景来获得统一的见解。换句话说,我们关注的不是“如何”而是“为什么”癌细胞表现出高有氧糖酵解。这最好是通过检查好氧糖酵解的后遗症,这可能带来一个选择优势。其中许多已经被考虑过,包括合成代谢底物的产生,糖酵解相对于呼吸的反应率,和抗氧化剂的产生。这里考虑的另一个结果是,好氧糖酵解导致乳酸产量高;导致细胞外空间酸化。事实上,低的细胞外pH会促进局部侵袭,促进转移,并抑制抗肿瘤免疫。在自然发生的癌症中,细胞外pH值低是无转移生存的一个强有力的阴性预后指标。此外,有研究表明,抑制细胞外酸中毒可抑制转移,提高抗肿瘤免疫。因此,我们认为,在癌症的体细胞进化过程中,过量的酸的产生为细胞提供了选择性优势。
Cancers progress through a series of events that can be characterized as
“somatic evolution.” A central premise of Darwinian evolutionary theory is that
the environment imparts pressure to select for species that are most fit within
that particular microenvironmental context. Furthermore, the rate of evolution
is proportional to both (1) the strength of the environmental selection and (2)
the phenotypic variance of the selected population. It is notable that, during
the progression of cancers from carcinogenesis to local invasion to metastasis,
the selective landscape continuously changes, and throughout this process, there
is increased selection for cells that have altered metabolic phenotypes:
implying that these phenotypes impart a selective advantage during the process
of environmental selection. One of the most prevalent selected phenotypes is
that of aerobic glycolysis, that is, the continued fermentation of glucose even
in the presence of adequate oxygen. The mechanisms of this so-called “Warburg
effect” have been well studied, and there are multiple models to explain how
this occurs at the molecular level. Herein, we propose that unifying insights
can be gained by evaluating the environmental context within which this
phenotype arises. In other words, we focus not on the “how” but the “why” do
cancer cells exhibit high aerobic glycolysis. This is best approached by
examining the sequelae of aerobic glycolysis that may impart a selective
advantage. Many of these have been considered, including generation of anabolic
substrates, response rates of glycolysis vis-à-vis respiration, and generation
of antioxidants. A further sequeala considered here is that aerobic glycolysis
results in a high rate of lactic acid production; resulting in acidification of
the extracellular space. Indeed, it has been shown that a low extracellular pH
promotes local invasion, promotes metastasis, and inhibits antitumor immunity.
In naturally occurring cancers, low extracellular pH is a strong negative
prognostic indicator of metastasis-free survival. Furthermore, it has been shown
that inhibition of extracellular acidosis can inhibit metastasis and promote
antitumor immunity. Hence, we propose that excess acid production confers a
selective advantage for cells during the somatic evolution of cancers.
Metabolism and Its Sequelae in Cancer Evolution and Therapy : The Cancer
Journal
https://journals.lww.com/journalppo/Abstract/2015/03000/Metabolism_and_Its_Sequelae_in_Cancer_Evolution.7.aspx
Origin of the Warburg Effect
Project Summary:
Eukaryotic cells have two mechanisms of energy (ATP) production: mitochondrial
respiration and glycolytic fermentation. Through the groundbreaking work of
Louis Pasteur, it was thought that the less efficient fermentation would ensue
only if there was insufficient oxygen to support respiration. This was the
predominant thought until about 100 years ago, when the Nobel Prize-winning
German Physician, Otto Warburg showed that cancer cells ferment glucose at a
high rate, even in the presence of adequate oxygen and sufficient mitochondrial
function (1). The reasons that cancer cells choose to use the less efficient
fermentative pathway over the more efficient respiration have remained a mystery
ever since. We believe that we have solved this mystery, and only need to
convince others. We have argued that as the efficiencies are so different, this
has nothing to do with energy production, where most have been focusing their
time. Instead, we argue, that it must have to do with the production of a
by-product of fermentation (2). We believe that this byproduct is lactic acid,
and have shown through experiments that: 1) acid production is associated with
increased cancer invasion (3, 4), 2) that neutralization of acid can prevent
invasion (5-10), and 3) that stimulation of acid production can promote invasion
(Russell, in prep). Hence, A) fermentation produces acid, B) acid production
improves the fitness and survival of cancer cells that do this, and C) cancers
that ferment are more likely to succeed and be predominant in those that we
detect (11-14).
https://lab.moffitt.org/gillies/research/tumor-ph/origin-of-the-warburg-effect/
References:
Warburg O, Posener K. Uber den stoffwechsel der carcinomzelle. Bioichem Z
1924;152:309-44.
Gillies RJ, Robey I, Gatenby RA. Causes and consequences of increased glucose
metabolism of cancers. J Nucl Med 2008;49 Suppl 2:24S-42S.
Estrella V, Chen T, Lloyd M, Wojtkowiak J, Cornnell HH, Ibrahim-Hashim A, et al.
Acidity generated by the tumor microenvironment drives local invasion. Cancer
research 2013;73(5):1524-35.
Gatenby RA, Gawlinski ET, Gmitro AF, Kaylor B, Gillies RJ. Acid-mediated tumor
invasion: a multidisciplinary study. Cancer research 2006;66(10):5216-23.
Ibrahim Hashim A, Cornnell HH, Coelho Ribeiro Mde L, Abrahams D, Cunningham J,
Lloyd M, et al. Reduction of metastasis using a non-volatile buffer. Clin Exp
Metastasis 2011;28(8):841-9.
Ibrahim-Hashim A, Abrahams D, Enriquez-Navas PM, Luddy K, Gatenby RA, Gillies
RJ. Tris-base buffer: a promising new inhibitor for cancer progression and
metastasis. Cancer Med 2017.
Ibrahim-Hashim A, Cornnell HH, Abrahams D, Lloyd M, Bui M, Gillies RJ, et al.
Systemic buffers inhibit carcinogenesis in TRAMP mice. J Urol
2012;188(2):624-31.
Ibrahim-Hashim A, Robertson-Tessi M, Enriquez-Navas PM, Damaghi M,
Balagurunathan Y, Wojtkowiak JW, et al. Defining Cancer Subpopulations by
Adaptive Strategies Rather Than Molecular Properties Provides Novel Insights
into Intratumoral Evolution. Cancer research 2017;77(9):2242-54.
Ribeiro MD, Silva AS, Bailey KM, Kumar NB, Sellers TA, Gatenby RA, et al. Buffer
Therapy for Cancer. Journal of nutrition & food sciences 2012;2:6.
Robey IF, Baggett BK, Kirkpatrick ND, Roe DJ, Dosescu J, Sloane BF, et al.
Bicarbonate increases tumor pH and inhibits spontaneous metastases. Cancer
research 2009;69(6):2260-8.
Gatenby RA, Gillies RJ. Why do cancers have high aerobic glycolysis? Nat Rev
Cancer 2004;4(11):891-9.
Gatenby RA, Gillies RJ. A microenvironmental model of carcinogenesis. Nat Rev
Cancer 2008;8(1):56-61.
Gatenby RA, Gillies RJ, Brown JS. Evolutionary dynamics of cancer prevention.
Nat Rev Cancer 2010;10(8):526-7.
Gillies RJ, Verduzco D, Gatenby RA. Evolutionary dynamics of carcinogenesis and
why targeted therapy does not work. Nat Rev Cancer 2012;12(7):487-93.