光动力灭活哺乳动物病毒和噬菌体
Photodynamic Inactivation of Mammalian Viruses and Bacteriophages
抽象
光动力学灭活(PDI)通过使用光敏剂来灭活微生物。自上个世纪的头几十年以来,通过光敏化使哺乳动物病毒和噬菌体失活已经成功应用。由于已知哺乳动物病毒对公众健康构成威胁并且噬菌体经常被用作哺乳动物病毒的模型,因此了解和理解其光活化中涉及的机制和光动力学程序是重要的。
本综述的目的是(i)总结迄今为止对噬菌体和哺乳动物病毒的光动力灭活所开发的主要方法,以及(ii)讨论和比较哺乳动物病毒PDI与噬菌体光灭活的现有技术水平,特殊重点关注最相关的机制,分子靶点和影响病毒灭活过程的因素。
关键词:噬菌体,哺乳动物病毒,光动力疗法,光敏剂,病毒光灭活过程
1.简介
人类通过各种途径暴露于致病病毒,并且病毒诱发的疾病的发展是常见的。
虽然通过开发良好的供水和基于卫生的程序来减少病毒性疾病的传播,但是病原性病毒仍然是人类和其他物种中许多疾病的致病因子。由病毒引起的最常见的人类疾病包括普通感冒(冠状病毒),流感(流感病毒),水痘(水痘带状疱疹病毒),唇疱疹(单纯疱疹病毒),肠胃炎和腹泻(杯状病毒,轮状病毒和腺病毒)[2, 3]。致病病毒还涉及严重疾病,如埃博拉病毒(埃博拉病毒),艾滋病(免疫缺陷病毒),禽流感和突发性急性呼吸道综合症(SARS)(SARS冠状病毒),它们也是癌症的确定病因(乳头瘤病毒) ,乙型肝炎和丙型肝炎病毒,爱泼斯坦 - 巴尔病毒,卡波西肉瘤相关疱疹病毒,人类T淋巴细胞病毒和默克尔细胞多瘤病毒[4]。
病毒在严重传染病中的重要性以及对病毒发病机制的复杂机制的日益增长的知识极大地促进了抗病毒药物的快速发展。因此,抗病毒药物的使用在过去几年中已大大增加,现在对几种病原性病毒的抗病毒药物的耐药性已得到充分证实[5,6,7,8,9,10]。此外,由于病毒具有遗传上的灵活性,它们可能会迅速发生突变,突变也不会出现意外,从而导致对常规抗病毒药物产生抗药性。因此,抗病毒药物的出现可能成为一个很大的问题,例如细菌相对于抗生素的耐药性。因此,需要不太可能引起耐药的替代方法。病毒的光动力学灭活(PDI)代表了满足该需要的有前景且廉价的潜在替代方案。
遵循病毒光灭活过程的简单和定量测定的可用性是重要的。传统的病毒定量技术,例如体外病毒培养,是耗时且劳动密集的过程。分子定量方法,例如核酸扩增程序,包括实时PCR,是快速且灵敏的,但仅检测病毒核酸并且不确定感染性。当最初评估不同光敏化合物的杀病毒特性时,噬菌体可用作哺乳动物病毒的替代物。其使用的原因是:(i)检测方法比哺乳动物病毒更简单,更快速和更便宜,避免了繁殖人类病原体所需的先进设施和设备; (ii)它们对人类无致病性; (iii)它们可以比大多数哺乳动物病毒生长到更高的滴度,因此增强了试验的灵敏度; (iv)噬菌体试验的结果可在接种后数小时内获得,而不是哺乳动物病毒传染性检测所需的天或周; (v)它们至少与哺乳动物病毒一样对环境因素和水处理具有抗性[48]。
已经证明包膜病毒对光动力学破坏比非包膜病毒更敏感[49,50]。由于大多数噬菌体是无包膜的,因此它们比包膜病毒更难以进行光灭活。一般而言,该特性使其成为评估病毒性PDI效率的良好指标。有效灭活无包膜噬菌体的PDI方案很可能对包膜哺乳动物病毒有效。
几种噬菌体用于光灭活研究,作为哺乳动物病毒的替代品,如MS2 [44],M13 [51,52],PM2 [53],Qβ[54,55,56],PRD1 [57],λ[58, 59],φ6[60],R17 [60],沙雷氏菌噬菌体kappa [61],T5 [62],T3 [63],T7 [57,64]和T4样[65,66,67,68],结果表明它们是能被有效光活化的。
2.抗菌PDI
PDI是一种简单且可控的基于活性氧(ROS)(自由基和单线态氧)产生的微生物灭活方法。这项技术需要氧气,光和光敏剂(PS)的联合作用,它吸收并利用光能产生这些ROS [69]。因此,光动力效应取决于多个变量,包括:PS的结构特征,PS和分子氧的浓度,以及所用光的性质(例如,波长,类型,剂量和注量率)[66,67, 69,70,71,72。任何这些参数的变化都会影响微生物光灭活的速率[66,67,73,74]。
PDI中使用的大部分PS来源于称为卟啉的四吡咯大环化合物。这些发色团及其类似物,如二氢卟酚和菌绿素,参与非常重要的生物学功能,如呼吸(血红素组)和光合作用(叶绿素和细菌叶绿素)(图1)。基于这些大环素,科学界得以发展许多合成类似物,如内消旋 - 四芳基卟啉,酞菁,泰克萨菲林,卟啉和卟啉,它们被证明具有非常有前途的特性,可用作PS(图2)[16]。此外,非四吡咯衍生物,如天然存在的金丝桃素,或合成染料如甲苯胺蓝O,玫瑰红,曙红,亚甲蓝(MB)和富勒烯,在许多PDI研究中被考虑(图3)[71]。
为了有效,用于病毒PDI的光敏剂必须与重要的病毒组分特异性结合,如脂质包膜(如果存在),蛋白质外壳或核酸[55]。
已经描述了哺乳动物病毒和噬菌体PDI的效率,用于卟啉衍生物,二氢卟酚衍生物,叶绿素衍生物,酞菁衍生物,金丝桃素,亚甲蓝,玫瑰红,部花青540,白兰碱和富勒烯衍生物(表1)。
此外,病毒PDI也被描述为酞菁衍生物[81],亚甲基蓝[53,62,91,92],甲苯胺蓝O [53,62,93],中性红[93],proflavine [93], azure B [53]和merocyanine 540 [45,47,94]。
3.光动力学灭活机制
PDI的机制基于PS从光吸收能量并将该能量转移到分子氧的能力。在黑暗中,PS的电子配置存在于所谓的基态。通过PS吸收适当波长的光子最初会导致PS分子的不稳定的电子激发态(该状态的寿命在10-9到10-6秒之间)[95] ]。然后激发的PS分子可以通过发射光(辐射通路 - 荧光)或通过系统间交叉衰变到基态,从而提供具有更长寿命(10-3到10s)的激发三重态[95]。此时,PS可以通过自旋反转然后通过磷光发射或通过非辐射过程达到基态。由于PS三重态的寿命较长,这种激发态也可以通过两种方式之一发生反应(图2):通过启动可直接产生活性氧(ROS)(I型通路)的光化学反应,或间接通过能量转移到分子氧(II型途径),导致单线态氧(1O2)的形成(图4)。这些事件提供了有毒物种,这些物种导致重要生物靶标的不可挽回的氧化损伤[1,69,95,96]。
3.1. I型和II型机制
I型机制涉及激发的PS和底物之间的氢原子提取或电子转移,产生自由基[方程式(1)和(2)]。这些自由基可以与氧反应形成活性氧物质,例如超氧自由基阴离子[等式(3)]。超氧化物在生物系统中不是特别反应,但是当质子化时,可以导致产生过氧化氢和氧[方程(4)和(5)]或高反应性羟基自由基[方程(6) - (8)] [98 ]。 II型光氧化在机理上比I型复杂得多,并且通常产品少得多[99]。在这个途径中,激发三重态PS(3PS *)可以将多余的能量转移到分子氧(3O2)并弛豫到基态(1PS),产生激发的单线态分子氧(1O2)[方程式(9)] [69 ]。 1O2具有高度亲电性,可与多种酶相互作用,导致蛋白质合成的抑制和DNA链的分子改变,从而改变遗传物质在其复制过程中的转录(诱变效应),并以此方式导致微生物死亡[等式(10)] [98,100]。与核酸和蛋白质一样,不饱和脂质也是1O2和自由基攻击的突出目标。除脂质本身外,脂质过氧化 - 随后的反应可以改变周围的蛋白质,核酸和其他分子[98]。因此,在微生物灭活过程中,对病毒包膜造成的不同种类的损伤很可能是重要的[13]。
I型和II型机制都可以同时或排他地发生,这些过程之间的比例取决于所用的PS以及底物和氧气的浓度[95]。 3PS *的有机底物和分子氧之间的竞争决定了反应途径是I型还是II型,并且在PDI过程中可以改变主要机制[101]。
3.2. 评估I型和II型机制的特异性参与
研究病毒性PDI的一个重要目标是在选定的PS存在下鉴定所涉及的机制类型(I型或II型)[102]。简单检测活性物质不一定解释特定PS诱导毒性作用的机制。通常更容易得出否定结论,即,如果不存在单线态氧,则它不能是负责光动力效应的反应性物质[103]。确定单线态氧(II型机制)或自由基(I型机制)是否参与光动力学过程的最简单方法是研究各种清除剂的抑制作用,即能够以高速率拦截这些ROS的化合物。一种假定的选择性方式[99,101,104]。
3.2.1. I型机制清除剂
当然,防御ROS的第一道防线是防止其形成。然而,一旦形成破坏性物种的拦截,以防止其进一步的有害反应,也是一种防御的失活策略。一般来说,自由基清除剂通过捐赠自己的一种电子来中和自由基物种。淬灭剂本身在电子捐赠之前和之后没有特别的毒性[105]。
三种不同类型的猝灭是可能的,其包括自由基特征的转移,形成反应性清除剂衍生的基团;捕获自由基,形成稳定或惰性的自由基阱;和模拟淬灭酶活性的分子。一般来说,清除剂分子可以阻止自由基形成或去除它们,然后才能破坏重要的分子成分[105]。
已经使用几种自由基清除剂来评估哺乳动物病毒和具有不同PS的噬菌体PDI的I型机制的特异性参与(表2)。
表2
3.2.1.1. 哺乳动物病毒PDI中的自由基
通常,自由基物种对研究的哺乳动物病毒的光活化几乎没有影响(表2)。事实上,可以观察到HSV [45,84,106],流感病毒[108],Semliki Forest病毒(SFV)和VSV [50]在不同的PS和清除剂如谷胱甘肽,D-存在下失活的速度。甘露醇,甘油,超氧化物歧化酶(SOD),过氧化氢酶和氢醌未受到显著影响。尽管这些数据表明自由基不是病毒灭活过程中的主要参与者,但不能排除I型反应途径的参与,正如使用merocyanine 540作为PS时谷胱甘肽和半胱胺提供的相当大的保护水平所表明的那样。用于灭活HSV-1 [45]。
3.2.1.2. 噬菌体PDI中的自由基
在自由基清除剂的存在下,一些噬菌体的光活化速率可以降低,这表明自由基物种在灭活过程中有贡献(表2)。特别地,据报道,在糖缀合的内消旋 - 四芳基卟啉存在下T7噬菌体光活化的抑制根据PS的结构和二甲基硫脲(DMTU)的浓度而变化[64,87]。事实上,meso-tetrakis(4-β-d-glucosylphenyl)porphyrin [64]和5,10,15-(4-β-d-半乳糖基苯基)-20-(五氟苯基)卟啉[87]的T7噬菌体PDI似乎主要由自由基物种介导,正如自由基清除剂DMTU的保护作用所揭示的,与5,10,15-(4-β-d-葡萄糖基苯基)-20-苯基卟啉的T7噬菌体光敏作用相反, I型机制的贡献明显较小。在约1.0mM DMTU时达到最高抑制;清除剂浓度的进一步增加并未降低光诱导的噬菌体失活的斜率。然而,尽管抑制了PS的功效,但DMTU并未完全抑制T7噬菌体PDI [64,87]。在L-半胱氨酸作为清除剂和作为PS的proflavine存在下,报道了T3噬菌体的类似结果。然而,多羟基化富勒烯对MS2的光活化速率不受SOD存在的影响,表明自由基物种的贡献可忽略不计,如超氧阴离子自由基[90]。在卟啉衍生物存在下,T4样噬菌体PDI也很少或不受自由基清除剂L-半胱氨酸和D-甘露醇的影响,导致自由基物种不是噬菌体PDI的主要参与者的结论[107] 。
3.2.2. II型机制淬火器
通常,化学单线态氧猝灭剂的作用涉及单线态氧与猝灭剂的反应,产生氧化产物。另一种可能性是通过物理猝灭使单线态氧失活至基态(3O2),通过能量或电荷转移实现,而不消耗氧气或产物形成[101,109]。蛋白质中组氨酸,色氨酸和酪氨酸的残留被认为是单线态氧的主要天然猝灭剂[110]。
已经使用几种单线态氧猝灭剂来评估具有不同PS的病毒PDI期间II型机制的特异性参与(表3)。
3.2.2.1. 哺乳动物病毒PDI中的单线态氧
单线态氧似乎是哺乳动物病毒中最重要的杀病毒活性介质(表3)。通过除氧或加入单线态氧猝灭剂,如β-胡萝卜素,咪唑,L-组氨酸或叠氮化钠,可显著抑制病毒光活化的速率[45,84,106,107,108]。金丝桃素可诱导HIV主要衣壳蛋白p24的光化学改变,其被叠氮化钠抑制,表明单线态氧损伤[111]。当使用部分花青素540 [45],酞菁衍生物[106]或玫瑰红[108]作为PS时,结果表明1O2是参与VSV光活化的主要细胞毒性物种,而I型反应物如羟基自由基则不太重要。
3.2.2.2. 噬菌体PDI中的单线态氧
考虑到在单线态氧猝灭剂存在下噬菌体的PDI,结果(表3)表明,在大多数研究的病例中,单线态氧是PDI诱导的毒性作用的重要介质。但是,不能排除自由基的参与。例如,在淬灭剂量依赖性模式中,通过叠氮化钠将MB对M13噬菌体的灭活从1.72log抑制到0.54log,直至浓度为3.5mM。然而,即使在叠氮化钠存在下也发生光失活,表明I型和II型机制都可能参与M13光灭活过程。在淬灭剂浓度范围为3.5至35mM的情况下,未观察到叠氮化钠保护作用,这可通过增加M13噬菌体光活化的速率来证明,此后达到平台期[52]。此外,单线态氧猝灭剂和过氧化氢的影响表明单线态氧是造成孟加拉孟氏菌噬菌体M13生物活性丧失的主要因素[51]。
在叠氮化钠存在下,5,10,15-(4-β-D-半乳糖基苯基)-20-(五氟苯基)卟啉光活化T7噬菌体的效率降低了38%[87]。该结果和在DMTU存在下获得的结果(表2)证明,对于该PS,两种机制在T7噬菌体光活化中起作用,其中I型是主要的。 Gábor等人获得了类似的结果。 [64]存在糖缀合的内消旋 - 四芳基卟啉衍生物作为PS并使用1,3-二苯基异苯并呋喃作为单线态氧猝灭剂。当用5,10,15,20-四(4-β-D-葡糖基苯基)卟啉光照处理T7噬菌体时,在1,3-二苯基异苯并呋喃存在下,失活率降低42%。当使用5,10,15-(4-β-D-葡糖基苯基)-20-苯基卟啉时,保护率显著增加(74%)。然后可以得出结论,PDI机制的类型取决于PS结构,对称衍生物主要通过自由基的产生发挥其毒性作用,不对称衍生物是否主要通过单线态产生[64]。然而,在Egyeki等人的研究中。 [87]使用相同的不对称5,10,15-(4-β-D-半乳糖基苯基)-20-(五氟苯基)卟啉作为PS,并且相同的噬菌体,毒性作用主要通过自由基的产生。除此之外,I型和II型过程的贡献是PS浓度依赖性的,并且在清除剂存在下测量的光灭活速率的总和小于没有清除剂的情况下测量的光灭活速率的总和。这个结果可能意味着单线态氧和羟基自由基介导的损伤之间的协同作用,或者也可以认为两种清除剂的效率都不是100%[64,87]。
最近的一项研究表明,在β-胡萝卜素存在下,多羟基化富勒烯悬浮液(40μM)的照射降低了PRD1和T7噬菌体的光失活率,证明了单线态氧的参与[57]。此外,当在卟啉衍生物和单线态氧淬灭剂叠氮化钠和L-组氨酸的存在下照射T4样噬菌体时,噬菌体失活的速率显著降低,表明单线态氧可能是杀病毒活性的重要介质。这些PS [107]。然而,根据获得的数据,不能排除其他失活机制[57,107]。
虽然关于噬菌体PDI中I型和II型机制的重要性的一些数据是不一致的,但一般来说,似乎II型途径比噬菌体PDI中的I型机制更重要。另一方面,只有少数研究关注单线态氧和自由基清除剂在病毒PDI相同方案下的同时作用[64,84,87,90,106,107]。
4.抗病毒PDI的分子靶点
由光动力学机制产生的短寿命ROS是导致关键分子靶标的损伤的原因[112]。不同的病毒靶标,如包膜脂质和蛋白质,衣壳和核心蛋白质以及核酸可被单线态氧和/或其他ROS(过氧化氢,超氧化物和羟基自由基)攻击,以实现感染性的丧失[84]。为了更好地理解光活化过程,PDI如何影响分子靶标的知识具有重要意义[113]。因此,对PS产生的有毒物种与脂质,蛋白质和核酸等关键生物分子之间的相互作用进行详细的光物理和光化学研究,对于光敏化过程效率的知识和预测至关重要[114]。然而,所进行的研究表明,PDI的主要目标取决于PS的化学结构,靶向病毒和光灭活机制[64]。
4.1. 核酸
根据病毒,核酸可以是DNA或RNA(单链或双链)。核酸的大小也根据病毒而变化。一些研究表明,DNA和RNA哺乳动物病毒和噬菌体都被PDI有效灭活。现在有相当多的信息表明像MB这样的PS可以结合并穿透病毒膜,因此它们会嵌入核酸。通过光激活,产生的ROS可以导致核酸的破坏,特别是在鸟嘌呤残基处,从而阻止病毒复制[115]。然而,取决于所涉及的机制,目标选择性存在差异:糖部分通常受自由基攻击(通过I型过程产生),鸟嘌呤残基是单线态氧的靶标(通过II型过程产生)[97]。
4.1.1. DNA损伤
从四个DNA碱基中,鸟嘌呤是最容易发生I型光敏反应的成分,因为它在DNA碱基中表现出最低的氧化电位,并且它是唯一可被单线态氧氧化的碱(II型)过程)[116]。
用MB和其他杂环染料处理病毒导致病毒DNA的损伤[53,65,75,76]通过碱基修饰或碱基缺失,单链断裂(SSB)或DNA与蛋白质的交联[ 34,75,81,88,117]。已知阳离子卟啉可通过插入碱基对或自堆叠而与核酸结合,由于鸟嘌呤残基的易氧化而在光灭活时诱导损伤[118,119,120]。
阳离子卟啉与DNA的结合可能是由于卟啉大环中带正电荷的取代基与DNA的带负电荷的磷酸氧原子之间的静电相互作用[120]。然而,卟啉与DNA结合不是有效光敏化的先决条件,因为游离卟啉在病毒灭活方面比DNA结合物种更有效[88]。这一观察结果与普遍认为的卟啉分子必须与光敏损伤部位紧密相关的观点相矛盾,这可以通过结合卟啉与游离卟啉相比单线态氧的量子产率较低来解释[ 88。 4.1.1.1。哺乳动物病毒DNA中的损伤病毒DNA被认为是MB和光的PDI的关键靶结构[93]。从用1.3μMBB处理的腺病毒中分离的DNA在Southern印迹分析中显示出涂片,表明随机DNA片段化[76]。 MB加光治疗HSV-1会导致DNA损伤并阻断DNA复制[121]。
4.1.1.2. 噬菌体DNA的损伤
已经提出T4噬菌体的内部组分是重要的靶标,因为MB需要穿过由其蛋白质衣壳形成的外部屏障以产生显著效果[65]。事实上,一些受照射的噬菌体仍然可以注射功能性遗传物质但却失去了形成斑块的能力,这表明它们的DNA被破坏了。蛋白质合成也严重受损[65]。用MB和铝酞菁四磺酸盐(AlPcS4)处理M13噬菌体导致DNA中的链断裂和哌啶不稳定键,这与感染性的丧失相关。这与病毒基因组病变可能导致致敏诱导的致死性的提议一致[81]。发现DNA链切割是MB浓度和光剂量依赖性。发现病毒灭活和DNA损伤是氧依赖性过程。然而,DNA损伤与PM2噬菌体感染性的丧失无关,如在转染研究中观察到的,其测量了提取的病毒DNA的感染性,表明来自MB处理的噬菌体的DNA与未处理的对照一样能够产生子代病毒。 [53]。观察到的DNA损伤与噬菌体感染性的丧失无关,可能不是病毒PDI的主要目标,因为100%的闭合环状DNA是从MB光处理的PM2噬菌体中回收的[53]。关于PDI对分离的病毒DNA的影响,在光照下用增加浓度的MB处理M13mp2 DNA产生增加量的8-氧代-7,8-二氢-2'-脱氧鸟苷(8-氧代),由单线态氧和可能由氧自由基产生的普遍加合物。在100μMBB下,DNA中每40个脱氧鸟苷残基产生1个残基的8-氧代。因此,用MB加光处理M13mp2 DNA导致脱氧鸟苷残基的推定改变,阻碍体外DNA合成的进展[116]。
4.1.2. RNA损伤
已经提出RNA是具有许多PS的病毒性PDI的关键因子,但是尚未报道RNA损伤与感染性丧失之间的相关性的直接证据,如用酞菁衍生物处理的VSV的情况[81]。在RNA中,对于DNA [71],鸟嘌呤被认为是光敏剂和光氧化的主要目标。
4.1.2.1. 哺乳动物病毒RNA的损伤
VSV基因组被30μgmL-1的叶绿素衍生物和红光照射损坏,导致RNA聚合酶活性降低多达85%,这可能是由于病毒RNA聚合酶复合物的损伤和98%抑制6小时内病毒RNA合成[77]。根据Moor等人的观点。 [82],VSV的RNA和/或RNA聚合酶复合物可能是AlPcS4和MB光活化的主要靶标。 MB和酞菁衍生物灭活VSV并抑制病毒包膜与Vero细胞的融合。与病毒灭活程度相比,抑制程度较小,表明非膜靶标,如病毒RNA,可能参与VSV光灭活。然而,没有关于RNA损伤与感染性丧失之间相关性的报道[81]。 MB和光对HIV-1的光活化导致其RNA的破坏[34]。
4.1.2.2. 噬菌体RNA的损伤
在MB加曝光后,QβRNA基因组显示出足够的致死性损伤以解释噬菌体的失活[122]。然而,噬菌体的蛋白质成分也在病毒PDI中发挥了一定作用[122]。在使用MB和玫瑰红作为PS的RNA光灭活的比较中,Schneider等。 [54]表明RNA中的8-氧代蛋白形成与R17和Qβ噬菌体失活之间存在因果关系。然而,描述了光动力学诱导的RNA损伤和病毒灭活之间没有直接关系[54]。单独的8-oxodguo形成或QβRNA的氧化损伤不能直接解释病毒的致死事件。用MB和光直接处理提取的噬菌体RNA导致感染性RNA测定中的活性丧失,但是如果噬菌体RNA用噬菌体本身用MB和光处理则存在更大的活性损失。结果表明,与其纯化的分离聚合物状态相比,QβRNA的感染活性受蛋白质相关病毒粒子状态的光灭活影响更大[92,122]。在不存在蛋白质的情况下,MB和光对纯化的RNA的灭活很可能是由于在结合MB的位点对RNA的氧化损伤而发生的,并且可能涉及氧化碱如8-氧鸟嘌呤或链断裂[122]。
尽管关注PDI在哺乳动物病毒和噬菌体的核酸中引起的损伤的报告数量减少,但可以得出结论,DNA和RNA都是病毒PDI的潜在靶标。然而,没有专门关注在相同PDI方案下对哺乳动物病毒和噬菌体的DNA和RNA诱导的损伤的研究。
4.2. 外结构
包膜病毒比无包膜病毒更快地失活,因为包膜结构的破坏通常伴随着病毒感染性的丧失[13,40,94,123,124]。光动力学反应对其包膜中和/或作为PS结合位点的主要包膜蛋白上存在的不饱和脂质造成的损害改变了它们的结构,避免了细胞感染和病毒复制[50,84]。然而,一些研究表明,无包膜病毒也可以通过PS的毒性作用有效地灭活[55,56,58,62,64,65,66,67,73,81,87,88,94,122]。
相对于无包膜病毒,包膜病毒对PDI的较高易感性表明病毒包膜可能是比核酸用于光敏化的更重要的靶标。它还表明包膜中存在的不饱和脂质以及主要包膜蛋白是重要的PDI靶标。然而,就目前所知,没有研究关注PDI后甚至其他病毒内部脂质后病毒包膜脂质的降解。然而,有许多关于PDI对病毒包膜蛋白以及其他核心蛋白的影响的研究。
包膜病毒比非包膜病毒更易于灭活的说法仅基于间接研究,该研究比较了包膜病毒和非包膜病毒的灭活结果。 PDI方案[30,36,45,77,81,82,83]中使用的包膜病毒仅测定其蛋白质改变,并且没有进行关于其脂质的额外实验工作。然而,Lytle等人获得的PDI结果。 [94]包膜的φ6噬菌体虽然间接地与文献中报道的关于脂质对病毒光活化过程的主要贡献的报道很好。
相对于PDI对蛋白质的降解,不同研究的结果表明,主要的损害是蛋白质交联的形成,其次是其他类型的损伤,包括蛋白质的损失,蛋白质分子构象的改变,质量和电荷,以及蛋白质条带强度的变化(表4)。
当在PS存在下用UV或可见光照射蛋白质时,可以观察到敏感氨基酸残基如半胱氨酸,L-组氨酸,酪氨酸,蛋氨酸和色氨酸的光氧化,以及肽链的共价交联,导致分子聚集体的形成[125,126],破坏它们正常的折叠构象,从而迫使它们进入影响其正常功能的其他构象[127]。事实上,交联/聚集材料的形成似乎是光敏介导的蛋白质氧化的主要后果[128],并且已经证明蛋白质交联的形成不是主要的光动力事件,而是敏感氨基酸残基的光氧化产物与蛋白质中的其他基团之间的二次反应[126]。
PS本身可以诱导一些酶折叠的改变,导致通常在蛋白质中屏蔽的一些氨基酸残基的暴露,以及通常暴露在分子中的其他氨基酸残基的屏蔽。这些蛋白质修饰导致其性质的变化,例如其几种氨基酸的溶解度,蛋白水解敏感性,吸光度和荧光发射。这些改变主要由过氧化氢和羟基自由基的产生介导,尽管也可能发生单线态氧介导的反应[129]。位于蛋白质表面的氨基酸以比埋在分子内部的残基快得多的速率被光氧化。如果蛋白质完全展开,易感氨基酸也可能受到攻击和光降解[103,130]。
4.2.1. 哺乳动物病毒外结构的损伤
已经表明,由于蛋白质损伤,包膜病毒可以被灭活[30,82,83,131]。然而,虽然据报道同样的治疗对某些无包膜病毒无效[83,131],但Wong等人的研究结果显示。 [85]表明,由于PDI对其病毒蛋白质的损伤,甚至无包膜病毒也可以被有效灭活(表4)。
HSV-1病毒包膜中的蛋白质被认为是部花青素540光敏化的主要靶标[45]。一些酞菁衍生物已被证明可诱导HSV蛋白中的交联,这可能是观察到的感染性丧失的原因[132]。通过SDS-PAGE蛋白质分析,具有酞菁衍生物的治疗后,揭示了在HSV-1包膜蛋白,这是由许多蛋白质的损失,交联材料在凝胶的顶部,并通过改变外观反射的不可逆变化在蛋白质的分子量和分子电荷。这些改变很可能导致HSV-1失活[83]。
在用3.75-30μLmL-1叶绿素衍生物和光处理的VSV中,没有检测到M蛋白条带,这伴随着G蛋白条带强度的降低[77]。在凝胶顶部也检测到大的蛋白质复合物,表明病毒PDI使蛋白质交联[77]。使用融合测定和蛋白质分析,显示MB和AlPcS4引起G蛋白强度的降低(已知其在将VSV与宿主细胞结合中起关键作用)并且略微降低。 M蛋白(基质蛋白)带和蛋白质交联的强度。然而,观察到的病毒蛋白质损伤无法解释VSV PDI [82]。 MBV和酞菁衍生物使VSV失活,从而抑制病毒包膜与Vero细胞的融合。然而,与病毒灭活程度相比,这种抑制程度较小(对于MB,43%抑制对比4.7 log或99.998%灭活)[81]。 Abe和Wagner [81]也发现MB和AlPcS4光处理后VSV G蛋白的相对丰度变化很小,他们还在SDS-PAGE分析中观察到了额外的蛋白质条带[81]。通过蛋白质印迹分析发现,在MB光处理后,HIV-1 p24和gp120蛋白的大小可能由于交联而改变[34]。然而,使用相同的PS,AlpcS4和MB,在导致完全VSV失活的条件下,观察到病毒蛋白的SDS-PAGE后蛋白质模式没有变化[135]。
Vzorov等人的结果。 [36]表明,当从重组载体表达时,卟啉抑制HIV Env蛋白的细胞融合活性(对于病毒进入以及诱导病毒细胞病变效应是重要的生物学功能)。这些结果表明,病毒Env蛋白是这些化合物的重要靶点[36]。
玫瑰红的流感病毒PDI改变了HA融合蛋白并导致蛋白质交联[108]。
蔷薇红痘病毒的光活化显着改变了痘苗病毒蛋白中的浓度和氧化组氨酸,表明失活归因于病毒蛋白的改变,而不是核酸[86]。
通过金丝桃素处理流感病毒和辛德毕斯病毒[30],导致包膜蛋白的广泛交联,这可能损害了病毒粘附和穿透宿主细胞的能力。
在低剂量PDI和MB浓度≥0.5μM后,无包膜肠道病毒71的蛋白质谱显著改变,如涂抹和几个蛋白质条带的消失所显示的[85]。然而,肠病毒71 PDI也是由于病毒基因组的损伤[85]。
4.2.2. 噬菌体外结构的损伤
尽管包膜噬菌体的可用数据有限,但与其他无包膜噬菌体相比,光诱导率显著提高[94]。由四种噬菌体的merocyanine 540,两种无脂质的无包膜噬菌体(phi X174和T7),无包膜的脂质噬菌体(PRD1)和带有外部脂蛋白包膜(phi 6)的包膜噬菌体的光灭活研究Lytle等人。 [94]。不同病毒的存活曲线清楚地证明了该PS对光灭活的不同水平的敏感性,其中phi 6最敏感,其次是T7(敏感性低21倍)。虽然PRD1和phi6都具有脂质组分,但只有phi6被PS光活化。因此,PRD1的内部脂质组分不足以允许部分花青素540的光活化.Hotze等人也观察到富勒烯衍生物的更高的失活速率。 [57]对于没有脂质的噬菌体(T7噬菌体)而不是PRD1噬菌体。噬菌体组成的差异是由于外部结构对单线态氧的不同抗性,因为PRD1具有带有内部脂质膜的双衣壳,而T7具有缺乏脂质的单个蛋白质衣壳,并且两个噬菌体都含有具有相似GC含量的双链DNA (T7为48%,PRD1为51%)[57]。
与通过FTIR光谱分析评估的PRD1和T7中的核酸和PRD1噬菌体中的脂质(≤13%)相对较小的效果相比,噬菌体蛋白质受到光敏化(30-92%)的显著影响[57]。较高的T7噬菌体失活与其蛋白质衣壳的更大损害一致。除此之外,SDS-PAGE分析进一步证明外源单线态氧诱导的衣壳蛋白的氧化交联可能是噬菌体失活的原因[57]。这种PS对MS2噬菌体失活的高度倾向(与PRD1和T7噬菌体相比)可能是由于其A蛋白受损,这对于感染其宿主大肠杆菌是必需的,因为它含有高反应性氨基酸,如蛋氨酸,半胱氨酸,组氨酸和酪氨酸,而不是对核酸的损害[57]。糖基化的取代卟啉导致蛋白质衣壳的结构变化和/或蛋白质-DNA相互作用的松弛,这可能是T7噬菌体失活的原因[64]。除了DNA结构的改变外,光处理还指出蛋白质结构和/或DNA-蛋白质相互作用的显著改变,这可能是光动力学失活的原因[87,88]。 DNA二级结构的改变也可能是噬菌体衣壳蛋白中光化学损伤和随后噬菌体颗粒破坏的结果。核心蛋白的光修饰也可导致噬菌体失活,即使DNA部分的一级结构得以保留,因为这些蛋白在感染和DNA穿透的早期事件中起重要作用[87]。 T7核蛋白的损伤是一个复杂的过程,显然噬菌体DNA和蛋白质衣壳都受光反应的影响[88]。在浓度增加的MB存在下照射Qβ噬菌体导致病毒RNA-蛋白质交联产物的量呈指数增加,这可能是病毒灭活中最重要的事件[92]。 Qβ噬菌体的RNA基因组在MB加上光照后含有足够的致死性病变,以解释所得的噬菌体失活。然而,数据还表明噬菌体的蛋白质成分以某种方式促成了噬菌体的失活[122]。 Qβ噬菌体的蛋白质成分参与光活化过程,因为蛋白质羰基化合物和RNA-蛋白质交联的形成是由MB加光曝光有效形成的[89]。交联形成与噬菌体失活的密切关联以及即使噬菌体基因组中的一个这样的交联将是致命的预期使得RNA-蛋白质交联损伤成为暴露于Qβ噬菌体的Qβ噬菌体的主要灭活损伤的强有力候选者。 MB和光[122]。
Abe和Wagner [81]观察到MB和AlPcS4光灭活后SDS-PAGE上M13噬菌体蛋白的少量改变。 Zupán等人的结果。 [136],表明四裂卟啉meso-四(1-甲基吡啶-4-基)卟啉不与衣壳蛋白相互作用,并且不会干扰蛋白质-DNA相互作用,即使它对intraphage DNA具有强烈的稳定作用。
5.抗PDI和恢复生存能力
在过去的几十年中,与抗生素一样,越来越多的抗病毒药物的开发为临床医生提供了以前无法获得的治疗选择。然而,随着抗病毒药物利用率的提高,抗病毒药物耐药性的发展得到了进一步的认识[1,7,137,138,139,140]。对于未能获得最大治疗益处的患者,以及可能传播抗性病毒的社区,耐药性对于医疗服务来说是昂贵的[9]。
现在迫切需要开发新颖,方便和廉价的措施来对抗抗生素无法治疗的感染并限制其他对抗生素抗性微生物的形成。光动力学技术可以提供一种方法来满足这种需要,无论是在治疗方面还是在消毒方面,通过与大多数抗菌药物的典型机制明显不同的机制[1,141,142]。
如前所述,光敏化涉及单线态氧和自由基物质的产生,其引起分子损伤。微生物是否能够对这些活性氧产生抗性仍然存在问题[143],因此,微生物对光敏化的抗性的发展仍然存在争议。到目前为止,微生物对PDI的抗性的发展尚不清楚,并且被认为是不可能开发的。一般而言,微生物菌株对PDI的抗性的发展应被视为不太可能发生的事件,因为该过程通常是多靶点的,ROS会对许多微生物组分造成损害,这与大多数抗菌剂的作用机制不一致。药物[139,144,145]。
与大多数常见抗菌药物相比,确保生存所需的分子改变数量太大,微生物需要多位点突变才能变得高度抗性,这一事件的概率明显低于单点突变,这通常是足够的赋予对小分子抑制剂的抗性[42,146]。抗微生物PDI的这种特殊性质对于慢性和/或复发性感染的重复治疗是重要的[139]。
与标准处理相比,抗菌PDI与可能需要施用数周以实现有效杀灭微生物的标准处理相比,在光照开始后不久,表现出严重且不可逆的微生物损害[66,68]。这种损害不允许产生或操作任何种类的抗毒或诱变机制。因此,抗菌PDI是非常有效的,到目前为止,还没有发现抗光敏突变体[68]。
5.1. 光敏化后哺乳动物病毒的抗性和恢复活力
North等人的数据。 [33]表明HIV叠氮胸苷(AZT)抗性菌株与AZT敏感菌株一样易于用苯并卟啉衍生物光敏化。这一发现并不令人惊讶,因为AZT(抑制逆转录)和光活化的苯并卟啉衍生物的作用机制是不同的。因此,在逆转录酶水平上发生的病毒突变不会影响光动力学破坏[33]。
关注可能发展的病毒抗性的研究非常稀少,并且对连续光动力学治疗后病毒活力的恢复知之甚少。
5.2. 光敏化后的噬菌体抗性和活力恢复
关于噬菌体,只有一项研究关注光敏化后病毒抗性的可能发展[68]。经过10个连续循环的光动力处理,T4样噬菌体,在三氯卟啉5-(五氟苯基)-10,15,20-三(1-甲基吡啶-4-基)卟啉(Tri-Py + -Me)存在下-PF)在白光照射下5.0μM,在实验过程中光活化速率没有变化,这意味着没有观察到抗性。如果发生噬菌体抗性,将在实验之间检测到噬菌体光活化效率的重要降低。除此之外,T4样噬菌体在照射120分钟后暴露于Tri-Py + -Me-PF后仍无法恢复其生存能力[68]。在Perdrau和Todd [12]的初步研究中,所有通过MB重新激活灭活的葡萄球菌噬菌体的尝试均未成功。
6.影响病毒性PDI的因素
6.1. 电荷数,对称性,中间取代基团的大小和光敏剂浓度的影响
已经表明PS的位置和结合位点高度依赖于结构和分子内电荷分布,是微生物PDI的重要因素[143,147]。
就分子结构而言,分子电荷在确定抗微生物活性中是重要的。带正电荷的PS通常更有效,并且可以比中性和阴离子PS分子更低的浓度起作用[144]。 PS分子上的正电荷似乎促进带正电荷的PS与病毒衣壳和包膜上的带负电位点之间的紧密静电相互作用,使PS定向于对特定微生物的稳定性和代谢至关重要的位点[44,147,148] ]。这种结合提高了光活化过程的效率。
阳离子PS光损伤可以通过PS结合或位于其附近的PS在核酸或病毒外部结构中诱导[136]。例如,带正电荷的PS更有可能导致核酸损伤,而中性或阴离子同源物则主要作用于微生物的外侧[149]。
内消旋取代基链的对称性和大小也影响光动力效应。具有相反带电基团的PS比具有相邻带电基团的PS更对称。 PS大环中相邻的正电荷应该由于静电排斥而导致分子畸变[150]。 PS的毒性可以通过在大环周边引入选择的取代基来调节。通过这种方式,可以操纵合成PS的物理化学性质,以增强其与病毒(例如病毒衣壳)的结构特征的相互作用,并最小化与质膜或哺乳动物细胞膜的相互作用[44]。
PS的两亲性质是影响PDI效率的另一个重要特征,可以通过在大周期外围引入足够的功能来调节,例如不同数量的正电荷,不对称电荷分布或芳烃侧链的引入[16,151] ]。
PS浓度也是必须考虑的重要参数,因为病毒PDI显示受PS浓度的强烈影响。增加PS浓度可减少实现完全病毒灭活所需的时间,从而提高特定PDI方案的效率[66]。
6.1.1. 哺乳动物病毒PDI
通过用1.0μM阴离子酞菁衍生物AlPcS4处理和用红光照射5分钟,可以获得VSV的完全失活(4.2log)。对于中性酞菁衍生物(Pc4),使用更低量的PS(4.5nM)和10分钟照射[82]实现完全失活(4log)。 PBS中VSV的失活与光照时间呈线性关系[82]。用纳摩尔浓度的金丝桃素和玫瑰红进行VSV,流感和仙台病毒的融合活性的灭活,并且绝对依赖于光并且随着照射时间的增加而增加[30]。通过阳离子对称卟啉内消旋 - 四(1-甲基吡啶-4-基)卟啉在10分钟内(> 3.7log)完全灭活PBS或血浆中的HAV。相比之下,用阴离子对称卟啉meso-四(4-磺酸苯基)卟啉灭活HAV至3.6 log需要90分钟[44]。灭活的速率和程度似乎随着内消旋取代基的性质而变化[44]。 HIV和VSV在金丝桃素和玫瑰红染色后以浓度依赖性方式丧失感染性[30]。
6.1.2. 噬菌体PDI
用中性卟啉衍生物观察到MS2噬菌体失活。然而,这需要比阳离子(1分钟)更长的照射时间(30分钟)[44]。中性糖基化取代的卟啉也可以显著光活化T7噬菌体[64,87]。通过在不同浓度(0.5,1.0和5.0μM)的六种阳离子卟啉存在下将噬菌体暴露于白光270分钟来实现T4样噬菌体PDI。结果表明,噬菌体光活化随PS浓度的变化而变化,浓度越高,效果最好[66]。 T4样噬菌体PDI也随着卟啉电荷的数量而变化,其中三 - 和四 - 四环卟啉衍生物在病毒灭活方面比双阳离子更有效,其使噬菌体灭活低于检测限。四 - 和三联卟啉衍生物(内消旋 - 四(1-甲基吡啶-4-基)卟啉和5-(五氟苯基)-10,15,20-三(1-甲基吡啶-4-基)卟啉)导致完全用40 W m-2照射270分钟后T4样噬菌体失活(约7log)[66]。当用658nm的光照射时,这种四环卟啉在λ噬菌体失活的另一项研究(减少7个对数)中显示出类似的结果[58]。在固定光照剂量下增加卟啉浓度会导致病毒灭活增加[58]。使用卟啉衍生物[87]也检测到浓度依赖性效应,但是超过2.0μM的PS,该过程饱和。卟啉浓度的进一步增加不会导致T7噬菌体的更高的失活速率。 PS的聚集和/或光漂白可能是解释[87]。测试具有不同烷基取代基的阳离子内消旋 - 四(1-烷基吡啶-4-基)卟啉衍生物的MS2噬菌体失活,但除5,10,15,20-四(4-磺酸基苯基)卟啉外,均显示出毒性即使没有光[44]。
在Gábor等人进行的一项研究中。 [64],发现具有对称糖基化基团的卟啉衍生物的效率是T7噬菌体失活过程中不对称基因的两倍。根据Costa及其同事[66]的研究,T4样噬菌体失活的速率也取决于内消旋取代基的亲脂特性。在卟啉核心的一个中间位置存在亲脂性芳基似乎在噬菌体失活中起重要作用,影响T4样噬菌体的速率和效率[66]。 Casteel等。 [44]还观察到MS2噬菌体在使用具有不同烷基取代基的PS时的光灭活速率的差异,并得出结论,灭活的速率和程度似乎随着内消旋取代基的性质而变化。
6.2. 不同光源和通量率对抗菌PDT的影响
PDT需要一个光源来激活PS,方法是将其暴露在特定波长的可见光或近可见光下[152]。用于PDT的光源还必须具有适当的光谱特征,其优先与PS的最大吸收波长范围一致,用于产生足够的ROS以产生有效的毒性效应[153]。
与化学的进步(与新的和更有效的PS的发现和合成相关)同时,在开发新光源方面也有很多活动,更适合光敏化过程。简而言之,这些包括通常基于固态激光二极管的用户友好型激光器,以及廉价的发光二极管(LED)和滤波的宽带灯[154]。
PS激活已经通过各种光源实现,例如电弧等离子体放电灯,金属卤素灯,幻灯片投影仪照明组件和各种激光器。对于较大区域的处理,使用非相干光源,例如钨丝,石英卤素,氙弧,金属卤化物和磷光体涂覆的钠灯。最近,诸如LED的非激光光源也已应用于PDT。这些光源便宜得多,体积小,重量轻且灵活性高,寿命可达数十万小时,并可制造成激活市售PS的波长[152,155,156,157,158,159]。
乍一看,有关PDT注量率效应的现有文献似乎是矛盾的。一些研究表明,在低注量率时损伤较小,其他研究表明在相同总注量下较高的注量率与较低的注射速率相比,有些研究表明没有影响注量率[152,157,158]。注量率的降低降低了氧气消耗速率,从而延长了可能形成单线态氧的半径,从而增加了光毒效应[159]。秦等人。 [160]表明,注量率的增加会增加微生物的损害,但是,它似乎有一个光子上限来观察这种效应。由于每个PS分子一次只能吸收一个光子,当光子数量绕过PS分子的数量时,PS将不再能够“过量”吸收光子,并且PDI的速率不会增加。事实上,如果光子的数量高于这个极限,抗菌效果会降低,因为悬浮液中的染料不会吸收所有多余的光[160]。 Schindl等人。 [161]提到光的生物效应取决于注量,而不管该剂量的传递时间。麦克莱恩等人。 [162]还表明,灭活光可以在短时间内以高辐照度施加或在较长时间内以较低辐照度施加。数值模型,假设在时间t发生的光动力损伤的速率与此时的注量率成比例,并且可以建立PS和氧的局部浓度。然而,根据该模型,如果在相同的时间段内应用,相对低的注量率几乎与高注量率源一样有效[163]。
光毒性效应与PS浓度和光通量之间也存在直接关联。随着PS浓度的降低,必须施加更多的光以实现相同的效果,反之亦然。较低剂量的PS需要较高的活化光能量,较高的能量密度需要较长的光照时间[96]。
6.2.1. 光对哺乳动物病毒PDI的影响
登革病毒灭活效果随着MB浓度的增加,光源功率密度的增加和光照时间的延长以及光照距离的减小而增加。这使得窄带宽光系统能够在更短的时间内以更大的距离杀死或灭活包膜病毒[74]。在MB存在下的VSV被红色(由272 W cm-2的LED入射光提供)或绿黄色光(由低压钠灯以165 W cm-2的注量率提供)快速灭活,但较慢通过白光(由一组荧光管提供,注量率为42 W cm-2)[46],表明较高的功率密度产生高的病毒灭活率而不是低的注量率。瓦格纳等人。 [164]还表明,在总剂量为1.8×104和3.2×104 J m-2的情况下,9 W m-2的红光分别使MB处理的VSV灭活6和≥7log。 VSV失活与红光照射的注量率呈线性关系[165]。
6.2.2. 光对噬菌体PDI的影响
就已知的噬菌体PDI而言,只有一项研究侧重于不同光源和功率密度的影响[67]。在这项研究中,阳离子卟啉衍生物(内消旋 - 四(1-甲基吡啶-4-基)卟啉和5-(五氟苯基)-10,15,20-三(1-甲基吡啶-4-基)卟啉),用不同光源(荧光PAR灯,太阳光和卤素灯),注量率范围为40 W m-2至1690 W m-2,有效地光活化无包膜噬菌体。测试的所有光源导致体细胞T4样噬菌体减少约7log。然而,灭活的速率和程度取决于光源,即当使用低注量率(40 W m-2)时和能量剂量时,当以较低的注量率传递光时效果明显更高。然而,取决于所使用的光源,需要不同的照射时间以使T4样噬菌体失活至检测极限。结果还表明,使用相同的注量率,T4样噬菌体失活的功效取决于所使用的光源,特别是当光以低注量率递送时。用5.0μMBB光照处理M13噬菌体,并以辐射剂量依赖性方式灭活[52]。 Kastury和Platz [58]表明,在固定光剂量下增加PS的浓度会导致病毒灭活增加,固定PS浓度下总光暴露的增加也是如此。 T1噬菌体的失活率随着注量率的增加而增加,表明样品与光源的距离是一个必须控制的变量[73]。在较高的PS浓度下,失活率达到最大值然后降低,因为染料的过滤效果降低了有效注量率[73]。在Lee等人的一个简单模型中。 [56],噬菌体存活率也可以被认为是光通量的指数减小的一部分(假设整个系统的注量均匀)。
7.结论
已经证明不同类型的PS在病毒PDI中的效率对于DNA或RNA病毒的不同类型的哺乳动物病毒和噬菌体,无论它们是包膜的还是非包膜的。尽管包膜病毒比非包膜病毒更容易灭活,但一些研究证实,无包膜的哺乳动物病毒和噬菌体可被PDI有效灭活。病毒核酸的类型尚未被描述为影响病毒光活化的重要因素,但就目前所知,没有研究专门关注DNA和RNA病毒的光活化行为。然而,与相同光敏条件下的DNA噬菌体相比,RNA噬菌体MS2对光灭活非常敏感。
已经阐明了涉及病毒光敏化过程的机制类型,并且确定单线态氧和自由基物种是有效病毒PDI的重要贡献者。然而,单线态氧的贡献似乎在哺乳动物病毒和噬菌体PDI中更为明显。然而,很少有研究同时比较病毒性PDI中涉及的两种类型机制(I型和II型)的贡献。无论是治疗哺乳动物病毒还是噬菌体,病毒光灭活的主要目标是外部结构。虽然有几项研究关于PDI对病毒蛋白的特异性影响,但对于不同类型的哺乳动物病毒和噬菌体,没有关于PDI对病毒脂质的特异性影响的研究。然而,已经清楚地表明包膜病毒比它们的非包膜病毒更容易灭活,这意味着病毒包膜上存在的脂质是病毒PDI的重要靶标。
PS可有效地将噬菌体灭活至检测极限,使其不能恢复活力,避免病毒抗性的发展。目前尚未知道哺乳动物病毒的特殊情况,但由于哺乳动物病毒和噬菌体的病毒靶标相同,因此预计在哺乳动物病毒的情况下不会产生抗性。除此之外,无论哺乳动物病毒是否对常规抗病毒剂敏感或具有抗性,抗病毒PDI同样有效。考虑到所有这些优点,用于病毒灭活的PDI可被视为常规抗病毒治疗的有希望的替代疗法,即用于血液和血液制品的消毒,防止病毒污染和用于治疗伤口和烧伤感染。病毒性PDI具有快速作用模式,并且还具有更经济和环保技术的额外益处,其可以成功地用于废水,饮用水和鱼类养殖水消毒的环境领域。
测试了不同的PS浓度和不同的光源和注量率,表明它们是必须在必须详细阐述病毒光敏化方案时必须考虑的重要PDI参数。哺乳动物病毒和噬菌体的灭活可以在微摩尔水平的PS浓度下实现,并且不同的光源同样有效,这取决于病毒暴露的最终剂量。除此之外,PS还可以通过添加不同的内消旋取代基和正电荷来调节,以促进它们与病毒的相互作用,使其对哺乳动物病毒和噬菌体PDI更有效。
对哺乳动物病毒和噬菌体获得的结果的相似性表明它们在进行病毒光灭活技术时表现出相似的行为:(i)用于病毒PDI的PS在哺乳动物病毒和噬菌体的光灭活中同样有效; (ii)哺乳动物病毒和噬菌体光敏化的机制涉及产生单线态氧(II型机制),其中自由基物种略有贡献(I型机制); (iii)单线态氧和自由基显示出影响病毒核酸以及哺乳动物病毒和噬菌体外表面中存在的蛋白质和脂质,后者受PDI的影响更大; (iv)哺乳动物病毒和噬菌体PDI的比率和程度也受相同因素的影响,如PS浓度和正电荷数,内消旋取代基的性质和位置,注量率和能量剂量。因此,重要的是坚持开展更多的PDI噬菌体研究,以澄清光敏后病毒PDI的某些方面,例如病毒核酸类型(DNA或RNA)对光活化效率的影响以及病毒抗性发展和活力恢复的可能性。使用噬菌体作为哺乳动物病毒光活化模型研究病毒PDI和抗病毒经典方法之间的协同效应也很重要。
Photodynamic Inactivation of Mammalian Viruses and Bacteriophages
Abstract
Photodynamic inactivation (PDI) has been used to inactivate microorganisms through the use of photosensitizers. The inactivation of mammalian viruses and bacteriophages by photosensitization has been applied with success since the first decades of the last century. Due to the fact that mammalian viruses are known to pose a threat to public health and that bacteriophages are frequently used as models of mammalian viruses, it is important to know and understand the mechanisms and photodynamic procedures involved in their photoinactivation. The aim of this review is to (i) summarize the main approaches developed until now for the photodynamic inactivation of bacteriophages and mammalian viruses and, (ii) discuss and compare the present state of the art of mammalian viruses PDI with phage photoinactivation, with special focus on the most relevant mechanisms, molecular targets and factors affecting the viral inactivation process.
Keywords: bacteriophages, mammalian viruses, photodynamic therapy, photosensitizer, viral photoinactivation process
Nomenclature
AlPcS4 Aluminum phthalocyanine tetrasulfonate AZT Azidothymidine BVDV Bovine viral diarrhea virus DMTU DimethylthioureaEMCVEncephalomyocarditis virus HAV Hepatitis A virus HBV Hepatitis B virus HCV Hepatitis C virus HIV Human immunodeficiency virus HPV Human papillomatosis virus HSV Herpes simplex virus LED Light emitting diode MB Methylene blue NM Not mentioned NQ Not quantified Pc4Silicon phthalocyanine PDI Photodynamic inactivation PS Photosensitizer ROS Reactive oxygen species SFV Semliki Forest virus SHV Suid herpes virusSOD Superoxide dismutase SSB Singlet strand breaks Tri-Py+-Me-PF5-(pentafluorophenyl)-10,15,20-tris(1-methylpyridinium-4-yl)porphyrin tri-iodide VSV Vesicular stomatitis virus VZV Varicella zoster virus 1O2 Singlet oxygen 3O2 Molecular oxygen1 PS Ground state photosensitizer3 PS* Triplet excited state photosensitizer
1. Introduction
Humans are exposed to pathogenic viruses through various routes and the development of viral-induced diseases is a common occurrence.
Although the transmission of viral diseases has been reduced by the development of good water supplies and hygienic-based procedures for a whole range of human activities [1], pathogenic viruses are still the causative agents of many diseases in humans and other species. The most usual human diseases caused by viruses include the common cold (coronaviruses), influenza (influenza viruses), chickenpox (varicella zoster virus), cold sores (herpes simplex virus), gastroenteritis and diarrhoea (caliciviruses, rotaviruses and adenoviruses) [2,3]. Pathogenic viruses are also implicated in serious diseases, such as Ebola (Ebola virus), AIDS (immunodeficiency viruses), avian influenza and sudden acute respiratory syndrome (SARS) (SARS-coronavirus), and they are also an established cause of cancer (papillomavirus, hepatitis B and C viruses, Epstein–Barr virus, Kaposi’s sarcoma-associated herpes virus, human T-lymphotropic virus, and Merkel cell polyomavirus) [4].
The enhanced implication of viruses in severe infectious diseases and the increasing knowledge about the complex mechanisms of viral pathogenesis have greatly contributed to the rapid development of antiviral drugs. Consequently, the use of antivirals has largely increased in the last years and resistance to antiviral drugs is now well documented for several pathogenic viruses [5,6,7,8,9,10]. Moreover, as viruses are genetically flexible, they may mutate quickly and mutations come as no surprises, leading to the development of resistance to conventional antiviral drugs. Consequently, the emergence of antiviral drug can become a great problem, such the resistance observed for bacteria relative to antibiotics. So, alternative methods unlikely to cause resistance are required. Photodynamic inactivation (PDI) of viruses represents a promising and inexpensive potential alternative to meet that need.
The sensitivity of viruses to photodynamic procedures was reported in the 1930s [11,12] but only within the last 30 years, with the development of new active molecules, namely photosensitizers (PS), and an increment of light technologies (lasers, LED, portability, etc.), have photodynamic techniques for the inactivation of viruses received growing attention [13]. Most of the clinical applications of PDI for treatment of infections have so far been directed to viral lesions [14]. Clinical PDI was first applied to the treatment of herpes infection in the early 1970s [15], particularly for herpes genitalis. Since then, a great variety of viruses has been effectively inactivated by photodynamic treatment using in vitro conditions [16] but, considering the clinical use of viral PDI, the procedures are limited to the treatment of papillomatosis, caused by human papillomatosis virus (HPV), like laryngeal papillomatosis [17] and epidermodysplasia verruciformis [18] and, in a small scale, to the treatment of viral complications in AIDS patients [19,20]. However, considerable progress has been made in the viral photodynamic disinfection of blood products. The major threat of viral contamination in blood and blood products comes from the immunodeficiency viruses (HIV) [21], hepatitis viruses [21,22,23], cytomegalovirus [23], human parvovirus B19 [24] and human T-cell lymphotropic virus type I and type II [23]. HIV has been inactivated in vitro following a photodynamic procedure [25,26,27,28,29,30,31,32,33,34,35,36,37,38,39]. The photoinactivation of hepatitis viruses in blood products has also been successfully tested against the hepatitis C virus (HCV) [37,40,41,42], hepatitis B virus (HBV) [43] and hepatitis A virus (HAV) [44]. Inactivation of cytomegalovirus [45], human parvovirus B19 [46] and human T-cell lymphotropic virus [47] in blood products was also efficiently achieved after photodynamic treatment.
The availability of a simple and quantitative assay to follow the viral photoinactivation process is important. Traditional viral quantification techniques, such as in vitro viral cultures, are time-consuming and labor-intensive processes. Molecular quantitative methods such as nucleic acid amplification procedures, including real time PCR, are rapid and sensitive but detect only viral nucleic acid and do not determine infectivity. When the virucidal properties of different photosensitizing compounds are initially evaluated, bacteriophages can be useful as surrogates of mammalian viruses. The reasons for their use are: (i) the detection methods are much simpler, faster and cheaper than those of mammalian viruses, avoiding the advanced facilities and equipment needed for propagating human pathogens; (ii) they are non-pathogenic to humans; (iii) they can be grown to higher titers than most mammalian viruses and, therefore, enhancing the sensitivity of the assay; (iv) the results of bacteriophages assays are available within several hours post-inoculation, instead of the days or weeks required by mammalian viruses infectivity-based assays; (v) they are at least as resistant as the mammalian viruses to environmental factors and to water treatment [48].
It has been shown that enveloped viruses are significantly more sensitive to photodynamic destruction than non-enveloped viruses [49,50]. As most of the bacteriophages are non-enveloped, they are more difficult to suffer photoinactivation than the enveloped viruses. In general, this property makes them good indicators to evaluate the efficiency of viral PDI. A PDI protocol that is effective to inactivate a non-enveloped phage will most likely be effective against enveloped mammalian viruses.
Several bacteriophages were used in photoinactivation studies as surrogates for mammalian viruses, e.g., MS2 [44], M13 [51,52], PM2 [53], Qβ [54,55,56], PRD1 [57], λ [58,59], φ6 [60], R17 [60], Serratia phage kappa [61], T5 [62], T3 [63], T7 [57,64] and T4-like [65,66,67,68], and the results show that they are effectively photoinactivated.
2. Antimicrobial PDI
PDI is a simple and controllable method for the inactivation of microorganisms based on the production of reactive oxygen species (ROS) (free radicals and singlet oxygen). This technology requires the combined action of oxygen, light and a photosensitizer (PS), which absorbs and uses the energy from light to produce those ROS [69]. Therefore, the photodynamic effects depend on multiple variables including: the structural features of the PS, the concentrations of PS and molecular oxygen, and the properties of the light used (e.g., wavelength, type, dose and fluence rate) [66,67,69,70,71,72]. Changes in any of these parameters will affect the rate of microbial photoinactivation [66,67,73,74].
The majority of PS used in PDI is derived from tetrapyrrolic macrocycles known as porphyrins. These chromophores and their analogs, such as chlorins and bacteriochlorins, are involved in very important biological functions, such as respiration (heme group) and photosynthesis (chlorophyll and bacteriochlorophyll (Figure 1). Based on these macrocycles, the scientific community was able to develop a number of synthetic analogs, such as meso-tetraarylporphyrins, phthalocyanines, texaphyrins, porphycenes and saphyrins, which proved to have very promising features for being used as PS (Figure 2) [16]. Also, non-tetrapyrrolic derivatives, such as the naturally occurring hypericin, or synthetic dyes like toluidine blue O, rose bengal, eosin, methylene blue (MB) and fullerenes, were considered in many PDI studies (Figure 3) [71].
In order to be efficient, photosensitizing agents used for viral PDI must bind specifically to vital viral components, such as lipid envelope (when present), the protein coat or to the nucleic acids [55].
Figure 1
An external file that holds a picture, illustration, etc.
Object name is viruses-04-01034-g001.jpg
Structure of some tetrapyrrolic macrocycles with natural occurrence.
Figure 2
An external file that holds a picture, illustration, etc.
Object name is viruses-04-01034-g002.jpg
Open in a separate window
Skeletons of some synthetic pyrrolic macrocycles used as photosensitizers.
Figure 3
An external file that holds a picture, illustration, etc.
Object name is viruses-04-01034-g003.jpg
Structure of some non-tetrapyrrolic photosensitizers.
The efficiency of mammalian viruses and bacteriophages PDI has been described for porphyrin derivatives, chlorin derivatives, chlorophyll derivatives, phthalocyanine derivatives, hypericin, methylene blue, rose bengal, merocyanine 540, proflavine, and fullerene derivatives (Table 1).
Table 1
Some PS used for mammalian viruses and bacteriophages PDI.
Photosensitizer Microorganism PDI Reference
Mammalian viruses
Hematoporphyrin derivative HSV-1 7 log [75]
HSV-1 <0.8 log [36]
Uroporphyrin Adenovirus 7 log [76]
Natural metalloporphyrin derivatives HIV-1 <0.8 log [36]
Chlorophyll derivatives VSV ~6 log [77]
7-despropionate-7-hydroxypropylmesopyropheophorbide a BVDV ~5 log [78]
EMCV ~0.2 log
Benzoporphyrin derivative monoacid ring A HIV-1 >4 log [33]
Glycoconjugated meso-tetraarylporphyrin derivatives HSV-1 6 log [79]
HSV-2 6 log
Metallo tetrasulfonated meso-tetraarylporphyrin derivatives HIV-1 ≤2 log [36]
Tetrasulfonated meso-tetraarylporphyrin derivatives HIV-1 ≤2 log [36]
HAV ~4 log [44]
meso-Tetrakis(1-methylpyridinium-4-yl)porphyrin HAV ~4 log [44]
meso-Tetrakis(1-butylpyridinium-4-yl)porphyrin HAV >3.8 log [44]
meso-Tetrakis(1-octylpyridinium-4-yl)porphyrin HAV >3.9 log [44]
Cationic β-vinyl substituted meso-tetraphenylporphyrin derivatives HSV-1 <3 log [80]
Aluminum dibenzodisulfophthalocyanine HIV-1 3.7 log [49]
Aluminum phthalocyanine tetrasulfonate HIV-1 >5 log [49]
VSV 4.2 log [82]
Adenovirus 4 log [76]
Silicon phthalocyanine derivative VSV 4 log [82]
Cationic phthalocyanines HIV-1 >5 log [49]
HSV-1 ≥5 log [83]
Hypericin HIV-1 NQ [30]
VSV 4-5 log
Influenza virus NQ
Sendai virus NQ
Methylene blue VSV 4.7 log [81]
HSV-1 5 log [84]
SHV-1 2.5 log [84]
HCV <2 log [41]
HIV-1 <2 log [41]
Adenovirus 7 log [76]
Dengue virus 5–6.4 log [74]
Enterovirus 71 ~8 log [85]
Vaccinia virus 5 log [86]
Phenothiazine derivatives VSV >4.4 log [60]
Rose bengal Vaccinia virus 5 log [86]
HIV-1 NQ [30]
VSV 4–5 log
Influenza virus NQ
Sendai virus NQ
Adenovirus 7 log [76]
Buckminsterfullerene SFV 7 log [50]
VSV 7 log
Merocyanine 540 HSV-1 5–6 log [45]
Bacteriophages
Glycoconjugated meso-tetraarylporphyrins T7 phage <3 log [64]
T7 phage <3.5 log [87]
Tetrasulfonated meso-tetraarylporphyrin derivatives MS2 phage >3.8 log [44]
meso-Tetrakis(1-methylpyridinium-4-yl)porphyrin λ phage <7 log [58]
MS2 phage >4.1 log [44]
T4 phage 7 log [66,67]
T7 phage <4 log [88]
5-(pentafluorophenyl)-10,15,20-tris(1-methylpyridinium-4-yl)porphyrin T4 phage 7 log [66,67,68]
5-(4-methoxicarbonylphenyl)-10,15,20-tris(1-methylpyridinium-4-yl)porphyrin T4 phage 7 log [66]
5-(4-carboxyphenyl)-10,15,20-tris(1-methylpyridinium-4-yl)porphyrin T4 phage 3.9 log [66]
5,10-bis(4-carboxyphenyl)-15,20-bis(1-methylpyridinium-4-yl)porphyrin T4 phage 1.4 log [66]
5,15-bis(4-carboxyphenyl)-10,20-bis(1-methylpyridinium-4-yl)porphyrin T4 phage 1.2 log [66]
5,10,15-tris(1-methylpyridinium-4-yl)-20-phenylporphyrin T7 phage 1.7 log [88]
Methylene blue Serratia phage kappa >4 log [61]
M13 phage 2.2 log [52,81]
f2 phage 5 log [56]
Qβ phage 7–8 log [56]
Qβ phage 7–8 log [89]
Phenothiazine derivatives R17 phage 4–7 log [60]
φ6 4–6.5 log
Rose bengal PRD1 phage ~3.5 log* [57]
T7 phage ~4.5 log*
Riboflavin λ phage <4 log [59]
Proflavine Serratia phage kappa 4 log [61]
T3 phage 7–11 log [63]
Polyhydroxylated fullerene MS2 phage ~4 log [90]
PRD1 phage ~2.5 log* [57]
T7 phage ~3.5 log*
MS2 phage ~5 log*
Open in a separate window
*log(N/N0)
Besides this, viral PDI has also been described for phthalocyanine derivatives [81], methylene blue [53,62,91,92], toluidine blue O [53,62,93], neutral red [93], proflavine [93], azure B [53] and merocyanine 540 [45,47,94].
3. Mechanisms of Photodynamic Inactivation
The mechanisms of PDI are based on the ability of the PS to absorb energy from light and transfer that energy to molecular oxygen. In the dark, the electronic configuration of a PS exists in the so-called ground state. The absorption, by the PS, of a photon at an appropriate wavelength initially leads to the production of an unstable, electronically-excited state of the PS molecule (the lifetime of this state ranges from 10−9 to 10−6 s) [95]. The excited PS molecule can then decay to the ground state by emission of light (radiative pathway - fluorescence) or by intersystem crossing, affording the excited triplet state which has a longer lifetime (10−3 to 10 s) [95]. At this point, the PS can reach the ground state either by spin inversion followed by phosphorescence emission, or by a non-radiative process. Due to the longer lifetime of the PS triplet state, this excited state can also react in one of two ways (Figure 2): by initiating photochemical reactions that can directly generate reactive oxygen species (ROS) (type I pathway), or indirectly by energy transfer to molecular oxygen (type II pathway), leading to the formation of singlet oxygen (Figure 4). These events afford toxic species which are responsible for the irreparable oxidative damages induced to important biological targets [1,69,95,96].
Figure 4
An external file that holds a picture, illustration, etc.
Object name is viruses-04-01034-g004.jpg
Open in a separate window
Schematic representation of the photosensitization process (adapted from [97]).
3.1. Type I and Type II Mechanisms
Type I mechanism involves hydrogen-atom abstraction or electron-transfer between the excited PS and a substrate, yielding free radicals [Equations (1) and (2)]. These radicals can react with oxygen to form active oxygen species, such as the superoxide radical anion [Equation (3)]. Superoxide is not particularly reactive in biological systems but, when protonated, can lead to the production of hydrogen peroxide and oxygen [Equations (4) and (5)] or highly reactive hydroxyl radicals [Equations (6)–(8)] [98]. Type II photooxidation is considerably less complex mechanistically than type I and in general there are far fewer products [99]. In this pathway, the excited triplet state PS (3PS*) can transfer the excess energy to molecular oxygen (3O2) and relax to its ground state (1PS) creating an excited singlet molecular oxygen (1O2) [Equation (9)] [69]. 1O2 is highly electrophilic and can interact with numerous enzymes, leading to the inhibition of protein synthesis and molecular alteration of DNA strands, which alters the transcription of the genetic material during its replication (mutagenic effect) and, in this way, leading to microbial death [Equation (10)] [98,100]. Like nucleic acids and proteins, unsaturated lipids are also prominent targets of 1O2 and free radical attack. Lipid peroxidation-ensuing reactions can alter surrounding proteins, nucleic acids and other molecules, in addition to the lipids themselves [98]. Therefore, it is likely that damage of different kinds caused to the viral envelope is important in the process of microbial inactivation [13].
An external file that holds a picture, illustration, etc.
Object name is viruses-04-01034-i001.jpg
Both type I and type II mechanisms can occur simultaneously or exclusively, and the ratio between these processes depends on the PS used and on the concentrations of substrate and oxygen [95]. The competition between organic substrates and molecular oxygen for the 3PS* determines whether the reaction pathway is type I or type II and the predominant mechanism can be changed during the course of the PDI process [101].
3.2. Evaluation of the Specific Involvement of Type I and Type II Mechanisms
An important goal in the investigation of viral PDI is to identify the type of mechanism involved (type I or type II) in the presence of a selected PS [102]. The simple detection of a reactive species does not necessarily explain the mechanism by which a specific PS induces the toxic effect. It is generally easier to draw a negative conclusion, i.e., if singlet oxygen is absent, it cannot be the reactive species responsible for the photodynamic effect [103]. The simplest approach for determining whether singlet oxygen (type II mechanism) or free radicals (type I mechanism) is involved in the photodynamic process is to study the inhibitory effects of various scavengers, i.e., compounds that can intercept these ROS at high rates and in a putatively selective manner [99,101,104].
3.2.1. Type I Mechanism Scavengers
A first line of defence against ROS is, of course, the protection against their formation. However, the interception of the damaging species once formed, to prevent it from further deleterious reactions, is also a deactivation strategy of defence. In general, free radical scavengers neutralize the radical species by donating one of their own electrons. The quenching agents themselves are not particularly toxic before and after the electron donation [105].
Three different types of quenching are possible, which include the transfer of the radical character with the formation of a reactive scavenger-derived radical; trapping of free radicals with the formation of a stable or inert free radical trap; and molecules which mimic quenching enzyme activities. In general, scavenger molecules either prevent free radicals from being formed or remove them before they can damage vital molecular components [105].
Several free radical scavengers have been used to evaluate the specific involvement of type I mechanism during mammalian viruses and bacteriophages PDI with different PS (Table 2).
Table 2
Free radical scavengers used in mammalian viruses and bacteriophages PDI.
PS Scavenger Microorganism Scavenger protection Reference
Mammalian viruses
Aluminum phthalocyanine tetrasulfonate Reduced glutathione VSV Little/no effect [106]
Mannitol Little/no effect
Glycerol Little/no effect
SOD Little/no effect
Polyhydroxylated fullerene Glutathione (2.0 mM) SFV no effect [50]
VSV no effect
Hydroquinone (2.0 mM) SFV no effect [50]
VSV no effect
Merocyanine 540 Glutathione (10 and 30 mmol L−1) HSV-1 30-50% [45]
Cysteamine (10 and 30 mmol L−1) 60-70%
SOD (1.5 to 29 U mL−1) no effect
Methylene blue Mannitol (100 mM) HSV-1 24% [84]
Glycerol (10 mM) 24%
SOD (300 U mL−1) 24%
Catalase (30 U mL−1) 24%
Bacteriophages
5,10,15-(4-β- d-glucosylphenyl)-20-phenylporphyrin DMTU (0.1–5.0 mM) T7 phage 44% [64]
5,10.15,20-Tetrakis(4-β- d-glucosylphenyl) porphyrin DMTU (0.1–5.0 mM) T7 phage 79% [64]
5,10,15-(4-β- d-galactosylphenyl)-20-(pentafluorophenyl)-porphyrin DMTU (0.1–5.0 mM) T7 phage 89% [87]
5-(pentafluorophenyl)-10,15,20-tris(1-methylpyridinium-4- yl)porphyrin D-mannitol (100 mM) T4 phage 20% [107]
Qβ no effect
L-cysteine (100 mM) T4 phage 9% [107]
5,10,15,20-tetrakis(1-methylpyridinium-4-yl)porphyrin D-mannitol (100 mM) T4 phage no effect [107]
Proflavine L-cysteine (0.025 M) T3 phage 75–80% [63]
Polyhydroxylated fullerene SOD MS2 phage no effect [90]
Open in a separate window
3.2.1.1. Free Radicals in PDI of Mammalian Viruses
Free radical species had, in general, little or no effect on the photoinactivation of the studied mammalian viruses (Table 2). In fact, it can be observed that the rate of inactivation of HSV [45,84,106], influenza virus [108], Semliki Forest virus (SFV) and VSV [50] in the presence of different PS and scavengers like glutathione, D-mannitol, glycerol, superoxide dismutase (SOD), catalase and hydroquinone was not significantly affected. Although this data suggest that free radicals are not major players in the viral inactivation process, the participation of type I reaction pathways cannot be ruled out, as was shown by the considerable level of protection afforded by glutathione and cysteamine when merocyanine 540 was used as PS for inactivation of HSV-1 [45].
3.2.1.2. Free Radicals in PDI of Bacteriophages
The photoinactivation rate of some bacteriophages can be reduced in the presence of free radical scavengers, suggesting a contribution of radical species in the inactivation process (Table 2). In particular, it was reported that the inhibition of T7 phage photoinactivation in the presence of glycoconjugated meso-tetraarylporphyrins varied according to the structure of the PS and the concentration of dimethylthiourea (DMTU) [64,87]. In fact, T7 phage PDI by meso-tetrakis(4-β-d-glucosylphenyl)porphyrin [64] and 5,10,15-(4-β-d-galactosylphenyl)-20-(pentafluorophenyl)porphyrin [87] seemed to be mainly mediated by free radical species, as revealed by the protection effect of free radical scavenger DMTU, contrary to T7 phage photosensitization by 5,10,15-(4-β-d-glucosylphenyl)-20-phenylporphyrin, which revealed a significantly smaller contribution from type I mechanism. The highest inhibition was reached at about 1.0 mM of DMTU; further increase in scavenger concentration did not decrease the slope of photoinduced inactivation of phages. However, in spite of inhibiting the efficacy of the PS, DMTU did not completely inhibit T7 phage PDI [64,87]. Similar results were reported for T3 phage in the presence of L-cysteine as the scavenger and proflavine as the PS. However, the photoinactivation rate of MS2 by a polydroxylated fullerene was not affected by the presence of SOD, suggesting a negligible contribution of radical species, such as the superoxide radical anion [90]. T4-like phage PDI was also little or not affected by the presence of free radical scavengers L-cysteine and D-mannitol in the presence of porphyrin derivatives, leading to the conclusion that free radical species are not major participants in phage PDI [107].
3.2.2. Type II Mechanism Quenchers
In general, the action of chemical singlet oxygen quenchers involves the reaction of singlet oxygen with the quenching agent, producing an oxidized product. Another possibility is the deactivation of singlet oxygen to ground state (3O2) by physical quenching, achieved by either energy or charge transfer, without consumption of oxygen or product formation [101,109]. Residues of histidine, tryptophan and tyrosine in proteins are considered to be major natural quenchers of singlet oxygen [110].
Several singlet oxygen quenchers have been used to evaluate the specific involvement of type II mechanism during viral PDI with different PS (Table 3).
Table 3
Singlet oxygen quenchers used on mammalian viruses and bacteriophage PDI.
PS Quencher Microorganism Quencher protection Reference
Mammalian viruses
Aluminum phthalocyanine tetrasulfonate Sodium azide VSV significant effect [106]
Tryptophan VSV Significant effect
Rose bengal β-carotene Influenza virus Significant effect [108]
Sodium azide
Hypericin Sodium azide HIV Significant effect [111]
Methylene blue Imidazole (5.0 and 10 mM) HSV-1 55–75% [84]
Bacteriophages
5,10,15-(4-β- d-galactosylphenyl)-20-(pentafluorophenyl)porphyrin Sodium azide (0.1–5.0 mM) T7 phage 38% [87]
5-(pentafluorophenyl)-10,15,20-tris(1-methylpyridinium-4-yl)porphyrin Sodium azide (100 mM) T4 phage 80% [107]
Qβ 39%
L-histidine (50 mM) T4 phage 74%
meso-tetrakis(1-methylpyridinium-4-yl)porphyrin Sodium azide (100 mM) T4 phage 90% [107]
L-histidine (100 mM) T4 phage 78%
5,10,15,20-Tetrakis(4-β- d-glucosylphenyl)porphyrin 1,3-diphenylisobenzofuran (0.1-5.0 mM) T7 phage 42% [64]
5,10,15-(4-β- d-glucosylphenyl)-20-phenylporphyrin 1,3-diphenylisobenzofuran (0.1-5.0 mM) T7 phage 74% [64]
Polyhydroxylated fullerene β-carotene T7 phage 69% [57]
PRD1 phage 56%
β-carotene (26 μM) MS2 phage 50–60% [90]
Rose bengal Sodium azide (3.5–35 mM) M13 phage 31% [52]
Open in a separate window
3.2.2.1. Singlet Oxygen in PDI of Mammalian Viruses
Singlet oxygen seems to be the most important mediator of virucidal activity (Table 3) on mammalian viruses. The rate of viral photoinactivation is significantly inhibited by oxygen removal or by addition of singlet oxygen quenchers, such as β-carotene, imidazole, L-histidine or sodium azide [45,84,106,107,108]. Hypericin may induce photochemical alterations on HIV major capsid protein p24, which are inhibited by sodium azide, suggesting that the damage results from singlet oxygen [111]. When merocyanine 540 [45], phthalocyanine derivatives [106] or rose bengal [108] were used as PS, the results suggest that 1O2 is the main cytotoxic species involved in VSV photoinactivation, while type I reactants such as hydroxyl radicals are less important.
3.2.2.2. Singlet Oxygen in PDI of Bacteriophages
Considering the PDI of bacteriophages in the presence of singlet oxygen quenchers, the results (Table 3) suggest that, in most of the studied cases, singlet oxygen is an important mediator of the toxic effect induced by PDI. However, the participation of free radicals cannot be ruled out. For instance, the inactivation of M13 bacteriophage by MB was inhibited from 1.72 log to 0.54 log by sodium azide in a quencher dose-dependent mode, up to a concentration of 3.5 mM. However, photoinactivation occurred even in the presence of sodium azide, suggesting that both type I and type II mechanisms may be involved in the M13 photoinactivation process. In the presence of quencher concentrations ranging from 3.5 to 35 mM, a sodium azide protective effect was not observed, as evidenced by increasing rates of M13 phage photoinactivation, reaching a plateau thereafter [52]. Also, the effect of singlet oxygen quenchers and of hydrogen peroxide indicated singlet oxygen as the main factor responsible for the loss of biological activity of bacteriophage M13 by rose bengal [51].
The efficiency of 5,10,15-(4-β-D-galactosylphenyl)-20-(pentafluorophenyl)porphyrin to photoinactivate T7 phage decreased in 38% in the presence of sodium azide [87]. This result, and the ones obtained in the presence of DMTU (Table 2), proved that for this PS, both mechanisms play a role in T7 phage photoinactivation, with type I being the predominant one. Similar results were obtained by Gábor et al. [64] in the presence of glycoconjugated meso-tetraarylporphyrin derivatives as PS and using 1,3-diphenylisobenzofuran as the singlet oxygen quencher. When T7 phage was phototreated with 5,10,15,20-tetrakis(4-β-D-glucosylphenyl)porphyrin, the rate of inactivation decreased 42% in the presence of 1,3-diphenylisobenzofuran. When 5,10,15-(4-β-D-glucosylphenyl)-20-phenylporphyrin was used, the rate of protection substantially increased (74%). It can then be concluded that the type of PDI mechanism depends on the PS structure, with the symmetric derivative exerting its toxic effect mainly via the generation of free radicals, whether the asymmetric derivative proceeds mainly by singlet production [64]. However, in the study of Egyeki et al. [87] using the same asymmetric 5,10,15-(4-β-D-galactosylphenyl)-20-(pentafluorophenyl)porphyrin as PS, and the same phage, the toxic effect occurred mainly via free radical generation. Besides this, the contribution of type I and type II processes was PS concentration-dependent and the sum of the photoinactivation rate measured in the presence of scavengers was smaller than the one measured without the scavengers. This result may imply a synergism between singlet oxygen and hydroxyl radical-mediated damages or it can also be supposed that the efficiency of neither scavenger is 100% [64,87].
A recent study showed that irradiation of polyhydroxylated fullerene suspensions (40 μM) in the presence of β-carotene reduced the photoinactivation rate of PRD1 and T7 phages, demonstrating singlet oxygen involvement [57]. Also, when the T4-like phage was irradiated in the presence of porphyrin derivatives and singlet oxygen quenchers sodium azide and L-histidine, the rate of phage inactivation was considerably reduced, suggesting that singlet oxygen may be an important mediator of the virucidal activity of these PS [107]. However, from the data obtained, other inactivation mechanisms cannot be excluded [57,107].
Although some data about the importance of the type I and II mechanisms in PDI of bacteriophages are discrepant, in general, it seems that the type II pathway is more important than the type I mechanism in phage PDI. On the other hand, there are only a few studies focusing on the simultaneous effect of singlet oxygen and free radicals scavengers under the same protocol of viral PDI [64,84,87,90,106,107].
4. Molecular Targets of Antiviral PDI
The short-lived ROS generated by photodynamic mechanisms are responsible for the damage induced to critical molecular targets [112]. Different viral targets, such as the envelope lipids and proteins, capsid and core proteins and the nucleic acid can be attacked by singlet oxygen and/or other ROS (hydrogen peroxide, superoxide and hydroxyl radicals) to achieve the loss of infectivity [84]. For a better understanding of the photoinactivation process, the knowledge of how the molecular targets are affected by PDI assumes a great importance [113]. For this reason, a detailed photophysical and photochemical study of the interactions between the toxic species generated by the PS and key biomolecules such as lipids, proteins and nucleic acids is essential for the knowledge and prediction of photosensitization process efficiency [114]. However, the studies performed show that the primary target of PDI depends on the chemical structure of the PS, the targeted virus and the mechanism of photoinactivation [64].
4.1. Nucleic Acids
Depending upon the viruses, the nucleic acid can be either DNA or RNA (single or double stranded). The size of the nucleic acid also varies depending on the viruses. Several studies have shown that both DNA and RNA mammalian viruses and phages are efficiently inactivated by PDI. There is now considerable information that PS like MB can bind to and penetrate viral membranes, whereupon they intercalate with nucleic acids. Upon activation by light, the generated ROS can cause the destruction of the nucleic acids, particularly at guanine residues, preventing viral replication [115]. However, there is a difference in target selectivity depending on the mechanism involved: sugar moieties are usually attacked by radicals (generated via type I process) and guanine residues are the targets of singlet oxygen (generated via type II process) [97].
4.1.1. DNA Damage
From the four DNA bases, guanine is the most susceptible component to suffer a type I photosensitization reaction, due to the fact that it exhibits the lowest oxidation potential among DNA bases and it is the only base that can be oxidized by singlet oxygen (type II process) [116].
The treatment of viruses with MB and other heterocyclic dyes resulted in the damage of viral DNA [53,65,75,76] either by base modification or base loss, single strand breaks (SSB), or cross-links of DNA with proteins [34,75,81,88,117]. It is known that cationic porphyrins can bind to nucleic acids via intercalation into base pairs or self-stacking, inducing lesions upon photoinactivation due to the easy oxidation of guanine residues [118,119,120].
The binding of cationic porphyrins to DNA is presumably due to the electrostatic interaction between the positively-charged substituents in the porphyrin macrocycle and the negatively charged phosphate oxygen atoms of DNA [120]. However, porphyrin binding to DNA is not a prerequisite for an efficient photosensitization, since free porphyrins are more effective in virus inactivation than the DNA-bound species [88]. This observation, which is in conflict with the generally accepted idea that the porphyrin molecule must be in close vicinity with the site of photosensitized damage, may be explained by the lower quantum yield of singlet oxygen by the bound porphyrin when compared with the free one [88].
4.1.1.1. Damages in the DNA of Mammalian Viruses
Viral DNA is thought to be a critical target structure for PDI by MB and light [93]. DNA isolated from adenovirus treated with 1.3 μM MB exhibited a smear in Southern blot analysis, indicative of random DNA fragmentation [76]. MB plus light treatment of HSV-1 gives rise to DNA damage and blocks DNA replication [121].
4.1.1.2. Damage in the DNA of Bacteriophages
An internal component of T4 phage has been suggested as an important target because MB needs to cross the outer barrier made by its protein capsids in order to produce a significant effect [65]. In fact, some of the irradiated phages could still inject functional genetic material but have lost their ability to form plaques, suggesting that their DNA was damaged. Protein synthesis was also severely impaired [65]. Treatment of M13 phage with MB and aluminum phthalocyanine tetrasulfonate (AlPcS4) caused strand breaks and piperidine-labile bonds in DNA, which is correlated with the loss of infectivity. This is in agreement with the proposal that lesions of the viral genome might be responsible for the lethality induced by sensitization [81]. DNA strand cleavage was found to be MB concentration and light dose dependent. Viral inactivation and DNA damage were found to be oxygen-dependent processes. However, DNA damage was not correlated with the loss of PM2 phage infectivity, as observed in transfection studies which measured the infectivity of the extracted viral DNA, indicating that DNA from MB-treated phage was just as capable of generating progeny virus as the untreated controls [53]. The observed DNA damage is not correlated with loss of phage infectivity and may not be the prime target of viral PDI, because 100% of closed circular DNA was recovered from the MB phototreated PM2 phage [53]. Concerning the effects of PDI on isolated viral DNA, treatment of M13mp2 DNA with increasing concentrations of MB, in the presence of light, yielded increasing amounts of 8-oxo-7,8-dihydro-2'-deoxyguanosine (8-oxodguo), a prevalent adduct produced by singlet oxygen and perhaps by oxygen free radicals. At 100 μM MB, 1 residue of 8-oxodguo was produced for every 40 residues of deoxyguanosine in DNA. Thus, treatment of M13mp2 DNA with MB plus light resulted in putative alterations at deoxyguanosine residues that impede the progression of DNA synthesis in vitro [116].
4.1.2. RNA Damage
RNA has been suggested to be a key factor in viral PDI with many PS, but direct evidence of a correlation between RNA damage and infectivity loss has not been reported yet, as is the case of VSV when treated with phthalocyanine derivatives [81]. In RNA, as for DNA [71], guanine is suggested as the major target for oxidation by photosensitizing agents and light.
4.1.2.1. Damage in the RNA of Mammalian Viruses
VSV genome was damaged by 30 μg mL−1 of a chlorophyll derivative and red light illumination which caused a decrease of as much as 85% in RNA polymerase activity, which can be due to damage in the viral RNA polymerase complex, and 98% inhibition of viral RNA synthesis in 6 hours [77]. According to Moor et al. [82], the RNA and/or the RNA polymerase complex of VSV might be a major target for its photoinactivation by AlPcS4 and MB. MB and phthalocyanine derivatives inactivated VSV and inhibited fusion of the virus envelope with Vero cells. The degree of inhibition was small compared to the extent of virus inactivation, suggesting that non-membrane targets, like the viral RNA, might be involved in VSV photoinactivation. However, there is no report of a correlation between RNA damage and loss of infectivity [81]. Photoinactivation of HIV-1 by MB and light lead to destruction of its RNA [34].
4.1.2.2. Damage in the RNA of Bacteriophages
Following MB plus light exposure, the Qβ RNA genome exhibited sufficient lethal lesions to account for phage inactivation [122]. However, the protein component of the phage also exerted some effect in viral PDI [122]. In a comparison of RNA photoinactivation using MB and rose bengal as the PS, Schneider et al. [54] suggested a causal relationship between 8-oxodguo formation in RNA and R17 and Qβ bacteriophage inactivation. However, no direct relationship between photodynamically induced RNA damage and viral inactivation was described [54]. 8-oxodguo formation or oxidative damage of Qβ RNA alone does not directly account for the lethal event of the virus. Directly treating extracted phage RNA with MB and light caused a loss of activity in the infectious RNA assay but there was a much greater loss of activity if the phage RNA was treated with MB and light in the phage per se. The results demonstrated that Qβ RNA infectious activity is significantly more affected by photoinactivation in its protein-associated virion state as compared with its purified isolated polymer state [92,122]. Inactivation of purified RNA by MB and light, in the absence of proteins, most likely occurs due to oxidative damage to the RNA at the site at which MB is bound and might involve oxidized bases such as 8-oxoguanine or strand breaks [122].
In spite of the reduced number of reports focusing on the damage induced by PDI in the nucleic acids of mammalian viruses and bacteriophages, it can be concluded that both DNA and RNA are potential targets of viral PDI. However, there are no studies specifically focusing on the damages induced to DNA and RNA of both mammalian viruses and bacteriophages under the same PDI protocol.
4.2. Outer Structures
Enveloped viruses are inactivated more rapidly than non-enveloped viruses because the destruction of the envelope structure is generally accompanied by loss of virus infectivity [13,40,94,123,124]. The damages caused by photodynamic reactions on unsaturated lipids present in their envelopes and/or on major envelope proteins, which act as PS binding-sites, modify their structure and avoid cell infection and virus replication [50,84]. However, some studies showed that non-enveloped viruses can also be efficiently inactivated by the toxic action of PS [55,56,58,62,64,65,66,67,73,81,87,88,94,122].
The higher susceptibility to PDI of enveloped viruses, relatively to non-enveloped viruses, indicates that the viral envelope may be a more important target than nucleic acids for photosensitization. It also indicates that the unsaturated lipids present in the envelope, as well as the major envelope proteins, are important PDI targets. However, as far as it is known, no studies focus on the degradation of viral envelope lipids after PDI or even on other viral internal lipids. There are, however, many studies about the effects of PDI on viral envelope proteins as well as on other core proteins.
The statement that enveloped viruses are more easily inactivated than non-enveloped ones is only based in indirect studies which compare the inactivation results of enveloped and non-enveloped viruses. The enveloped viruses used in PDI protocols [30,36,45,77,81,82,83] were only assayed for their protein alterations and no additional experimental work was done concerning their lipids. However, the results of PDI obtained by Lytle et al. [94] with the enveloped φ6 phage, although indirectly, are in good accordance with what is reported in the literature about the major contribution from lipids for the viral photoinactivation process.
Relative to proteins degradation by PDI, the results of different studies showed that the main damage is the formation of protein cross-links, followed by other types of damage, which include loss of proteins, alterations in protein molecular conformation, mass and charge, and alterations in protein band intensity (Table 4).
When proteins are irradiated with UV or visible light in the presence of a PS, photooxidation of sensitive amino acid residues such as cysteine, L-histidine, tyrosine, methionine and tryptophan, and covalent cross-linking of peptide chains can be observed, leading to the formation of molecular aggregates [125,126], disrupting their normal folding conformation, thus forcing them into other conformations that affect their normal functioning [127]. In fact, the formation of cross-linked/aggregated material appears to be a major consequence of photosensitized-mediated protein oxidation [128], and it has been demonstrated that the formation of protein cross-links is not a primary photodynamic event, but a secondary reaction between the photooxidation products of sensitive amino acid residues and other groups in the protein [126].
The PS per se can induce alterations in the folding of some enzymes, leading to the exposure of some amino acid residues normally shielded in the protein, and to the shielding of others usually exposed in the molecule. These protein modifications lead to changes in properties such as solubility, proteolytic susceptibility, absorbance, and fluorescence emission of several of their amino acids. These alterations are mainly mediated by hydrogen peroxide and hydroxyl radical generation, although singlet oxygen mediated reactions could also occur [129]. The amino acids located in the surface of the protein are photooxidized at a much faster rate than the residues buried in the interior of the molecule. If a protein is completely unfolded, susceptible amino acids may also be attacked and photodegraded [103,130].
Table 4
Degradation of viral outer structures after mammalian viruses and bacteriophages PDI.
Virus Type of damage PS Reference
Enveloped-mammalian viruses
HSV-1 Viral envelope (reduced ability to adhere to and penetrate host cells) Merocyanine 540 [45]
Viral envelope (prevention of viral adsorption and host penetration) Phthalocyanine derivatives [131]
Glycoprotein D; loss of proteins; dimerization; protein cross-links; alterations in protein molecular mass and charge Phthalocyanine derivatives [83]
HSV-2 Viral envelope (prevention of viral adsorption and host penetration) Phthalocyanine derivatives [131]
HSV Protein cross-links Phthalocyanine derivatives [132]
VZV Viral envelope (prevention of viral adsorption and host penetration) Phthalocyanine derivatives [131]
HIV Major capsid protein p24 Hypericin [111]
HIV-1 Loss of infectivity; loss of fusion function; membrane proteins cross-links Hypericin [30]
Loss of infectivity; loss of fusion function; membrane proteins cross-links Rose bengal [30]
p24 and gp120 proteins; protein cross-links MB [34]
Inhibition of cell fusion activity of Env proteins Natural and sulfonated tetraarylporphyrins [36]
VSV Loss of infectivity; loss of fusion function; cross-linking of G and M proteins Hypericin [30]
Loss of infectivity; loss of fusion function; cross-linking of G and M proteins Rose bengal [30]
Inhibition of fusion of the envelope to Vero cells; G protein MB [81]
Inhibition of fusion of the envelope to Vero cells; G protein Aluminum phthalocyanine tetrasulfonate [81]
G and M proteins; protein cross-links Phthalocyanine derivatives [82]
G, M, L and N proteins; protein cross-links Chlorophyll derivatives [77]
Influenza virus Loss of infectivity; loss of fusion function; cross-linking of G and M proteins Hypericin [30]
Loss of infectivity; loss of fusion function; cross-linking of G and M proteins Rose bengal [30]
Loss of infectivity; HA fusion protein; protein cross-links Rose bengal [108]
Sendai virus Loss of infectivity; loss of fusion function; cross-linking of G and M proteins Hypericin [30]
Loss of infectivity; loss of fusion function; cross-linking of G and M proteins Rose bengal [30]
Vaccinia virus Histidine residues in virus proteins Rose bengal [86]
Human cytomegalovirus Viral envelope (reduced ability to adhere to and penetrate host cells) Merocyanine 540 [45]
Sindbis virus Viral envelope (reduced ability to adhere to and penetrate host cells) Merocyanine 540 [47]
Viral capsid protein Hypericin [133]
Friend erythroleukemia virus Viral envelope (reduced ability to adhere to and penetrate host cells) Merocyanine 540 [134]
Non-enveloped mammalian viruses
Adenovirus Not damaged Phthalocyanine derivatives [131]
Enterovirus 71 Appearance/disappearance of protein bands; increase of the protein band intensity Methylene blue [85]
T7 phage Protein capsid; loosening of the protein-DNA interaction Glycoconjugated meso-tetraarylporphyrins [64]
Capsid and core proteins; loosening of protein-DNA interaction Glycoconjugated meso-tetraarylporphyrins [87]
Capsid proteins; protein cross-links meso-Tetrakis(1-methylpyridinium-4-yl)porphyrin [88]
Capsid proteins; protein cross-links Polyhydroxylated fullerene [57]
M13 phage Coat protein Methylene blueAluminum phthalocyanine tetrasulfunate [81]
PRD1 phage Capsid proteins; protein cross-links; phospholipids (less affected) Polyhydroxylated fullerene [57]
Qβ phage Coat and maturation (A) proteins; formation of protein carbonyls; RNA-protein cross-links Methylene blue [89]
RNA-protein cross-links Methylene blue [92]
MS2 phage A protein Polyhydroxylated fullerene [57]
4.2.1. Damage on Mammalian Viral Outer Structures
It has been shown that enveloped viruses can be inactivated due to protein damage [30,82,83,131]. However, while the same treatment is reported to be ineffective against some non-enveloped viruses [83,131], the results from Wong et al. [85] showed that even a non-enveloped virus can be efficiently inactivated due to the damage induced by PDI to its viral proteins (Table 4).
The proteins in the viral envelope of HSV-1 were considered to be major targets of merocyanine 540 photosensitization [45]. Some phthalocyanine derivatives have been shown to induce cross-links in HSV protein that might be responsible for the observed loss of infectivity [132]. Protein analysis by SDS-PAGE, after treatment with phthalocyanine derivatives, revealed irreversible changes in the HSV-1 envelope proteins, which were reflected by the loss of many proteins, the appearance of cross‑linked material on the top of the gel and by alterations in the molecular mass and molecular charge of the proteins. These alterations contribute, in all likelihood to HSV-1 inactivation [83].
In VSV treated with 3.75–30 μL mL−1 of chlorophyll derivatives and light, the M protein band was not detected, which was accompanied by a decrease in the intensity of the G protein band [77]. Large complexes of proteins were also detected on the top of the gel, indicating that viral PDI cross-linked the proteins [77]. Using a fusion assay and protein analysis, it was shown that MB and AlPcS4 caused a decrease in the intensity of the G-protein (which is known to play a crucial role in binding VSV to the host cell) band and a slight decrease in the intensity of M protein (matrix protein) band and protein cross-links. However, the observed damage in viral proteins could not account for VSV PDI [82]. VSV was inactivated by MB and phthalocyanine derivatives, which inhibited the fusion of the virus envelope to Vero cells. However, the degree of this inhibition was small compared to the extent of virus inactivation (43% inhibition vs. 4.7 log or 99.998% inactivation, for MB) [81]. Abe and Wagner [81] also found few changes in the relative abundance of VSV G protein after MB and AlPcS4 phototreatment, and they also observed additional protein bands on SDS-PAGE analysis [81]. It was found, by Western blot analysis, that HIV-1 p24 and gp120 proteins were altered in size, possibly due to cross-linking, after MB phototreatment [34]. However, using the same PS, AlpcS4 and MB, no changes in protein patterns after SDS-PAGE of the viral proteins were observed, under conditions that caused complete VSV inactivation [135].
The results from Vzorov et al. [36] indicated that the porphyrins inhibited the cell fusion activity of HIV Env proteins (a biological function that is important for viral entry as well as induction of viral cytopathic effects) when expressed from recombinant vectors. These results showed that the viral Env protein is an important target of these compounds [36].
PDI of influenza virus by rose bengal altered the HA fusion protein and led to protein cross-links [108].
Photoinactivation of vaccinia virus with rose bengal significantly altered the concentration and oxidized histidine in vaccinia virus protein, suggesting that inactivation was attributed to alterations in viral proteins, as opposed to nucleic acids [86].
Treatment of of influenza and Sindbis viruses by hypericin [30], lead to an extensive cross-linking of the envelope proteins, which may have impaired the capacity of the viruses to adhere to and penetrate the host cells.
The protein profile of the non-enveloped enterovirus 71 was considerably altered after a low dose PDI and a MB concentration ≥0.5 μM, as revealed by a smearing and the disappearance of several protein bands [85]. However, enterovirus 71 PDI was also due to damages in the viral genome [85].
4.2.2. Damages on Bacteriophage Outer Structures
In spite of the limited available data for enveloped bacteriophages, substantially higher photoinactivation rates compared with other non-enveloped phages were described [94]. The photoinactivation by merocyanine 540 of four bacteriophages, two non-enveloped phages without lipids (phi X174 and T7), a non-enveloped phage with lipids (PRD1), and an enveloped phage with an external lipoprotein envelope (phi 6) was studied by Lytle et al. [94]. The survival curves of the different viruses clearly demonstrated different levels of sensitivity to photoinactivation by this PS, with phi 6 being the most sensitive, followed by T7 (21-fold less sensitive). While both PRD1 and phi 6 have lipid components, only phi 6 was photoinactivated by the PS. Thus, the internal lipid components of PRD1 were not sufficient to allow photoinactivation by merocyanine 540. A higher inactivation rate with a fullerene derivative was also observed by Hotze et al. [57] for a phage without lipids (T7 phage) than for PRD1 phage. The dissimilarities in phage composition resulted from differential resistance to singlet oxygen by the outer structures, since PRD1 has a double capsid with an internal lipid membrane, whereas T7 has a single proteinaceous capsid lacking lipids, and both phages contain double stranded DNA with similar GC content (48% for T7 and 51% for PRD1) [57]. Phage proteins were significantly affected by photosensitization (30–92%) when compared to the relatively smaller effect on nucleic acids in both PRD1 and T7, and lipids in PRD1 phage (≤13%), as assessed by FTIR spectra analysis [57]. The higher T7 phage inactivation is consistent with greater damage to its proteinaceous capsid. Besides this, SDS-PAGE analysis further evidenced that oxidative cross-linking of capsid proteins induced by exogenous singlet oxygen is the likely cause of phage inactivation [57]. The high propensity for MS2 phage inactivation by this PS (compared to PRD1 and T7 phages) possibly arises from damage to its A protein, which is necessary for infecting its host Escherichia coli since it contains highly reactive amino acids such as methionine, cysteine, histidine, and tyrosine and not to damages to the nucleic acid [57]. Glycosylated substituted porphyrins led to structural changes at the protein capsid and/or loosening of the protein-DNA interaction, which can be responsible for T7 phage inactivation [64]. Besides of the alteration of the DNA structure, the phototreatment pointed to significant alterations in the protein structure and/or in the DNA-protein interaction, which may be the cause of photodynamic inactivation [87,88]. The alterations in the DNA secondary structure might also be the result of photochemical damage in phage capsid proteins and consequent disruption of the phage particle. Photomodification of core proteins can also lead to phage inactivation, even if the primary structure of the DNA part is preserved, since these proteins play an important role in the early events of infection and DNA penetration [87]. The damage of T7 nucleoprotein is a complex process and clearly both phage DNA and protein capsid are affected by photoreactions [88]. Irradiation of Qβ bacteriophage in the presence of increasing concentrations of MB resulted in exponentially increasing amounts of viral RNA-protein cross-linkage products, and this is probably the most important event in viral inactivation [92]. The RNA genome of Qβ bacteriophage contained sufficiently lethal lesions following MB plus light exposure to account for the resulting phage inactivation. Nevertheless, the data also indicate that the protein component of the phage somehow contributes to the inactivation of the phage [122]. The protein component of Qβ phage is involved in the process of photoinactivation because the formation of protein carbonyls and RNA-protein cross-links were efficiently formed by MB plus light exposure [89]. The close correlation of cross-link formation with phage inactivation and the expectation that even one such cross-link in a phage genome would be lethal makes the RNA-protein cross-link lesion a strong candidate for the primary inactivating lesion of Qβ phage exposed to MB and light [122].
Little alteration of M13 phage proteins on SDS-PAGE after MB and AlPcS4 photoinactivation was observed by Abe and Wagner [81]. The results of Zupán et al. [136], suggested that the tetracationic porphyrin meso-tetrakis(1-methylpyridinium-4-yl)porphyrin did not interact with capsid proteins and did not disturb protein-DNA interaction, even if it has a strong stabilization effect on the intraphage DNA.
5. Resistance to PDI and Recovery of Viability
The development of increasing numbers of antiviral agents over the past decades, in the same way as with antibiotics, has provided the clinician with therapeutic options previously unavailable. With the increasing utilization of antiviral drugs, however, has come an enhanced appreciation of the development of antiviral resistance [1,7,137,138,139,140]. Drug resistance is costly to the health service, to the patient who fails to gain maximum therapeutic benefit, and for the community in which resistant viruses may be spread [9].
There is now an urgent need for the development of novel, convenient and inexpensive measures for combating antimicrobial-untreatable infections and limiting the development of additional antimicrobial resistant microorganisms. Photodynamic technology may provide one approach to meet this need, both in terms of therapy and in terms of sterilization, by a mechanism that is markedly different from that typical of most antimicrobials [1,141,142].
As mentioned before, photosensitization involves the generation of singlet oxygen and free radical species, which cause molecular damage. Whether microorganisms could develop resistance to these active oxygen species is still questionable [143] and, consequently, the development of microbial resistance to photosensitization is still under debate. Until now, the development of microbial resistance to PDI is not known and is thought very improbable to be developed. In general, the development of resistance to PDI by microbial strains should be considered as an unlikely event since this process is typically multi-target, with ROS causing damage to many microbial components, which is at a variance with the mechanism of action of most antimicrobial drugs [139,144,145]. In contrast to most common antimicrobials, the number of molecular alterations required to ensure survival would be too great and the microorganism would require multi-site mutations to become highly resistant, an event with significantly lower probability than single-site mutations, which is often sufficient for conferring resistance to small-molecule inhibitors [42,146]. This particular property of antimicrobial PDI is important regarding the repeated treatment of chronic and/or recurrent infections [139].
Antimicrobial PDI, when compared to standard treatments which may require application for several weeks to achieve an effective killing of the microorganism, shortly after initiation of light exposure, exhibits serious and irreversible damage of microorganisms [66,68]. This damage does not allow the creation or operation of any kind of anti-drug or mutagenic mechanism. Antimicrobial PDI is therefore very effective and, up until now, no photosensitization-resistant mutants have been found [68].
5.1. Resistance of Mammalian Viruses and Recovery of Viability after Photosensitization
Data from North et al. [33] show that HIV azidothymidine (AZT)-resistant strains were as susceptible as the AZT-sensitive ones to photosensitization with a benzoporphyrin derivative. This finding comes as no surprise since the mechanisms of action of AZT (inhibition of reverse transcription) and light-activated benzoporphyrin derivative are different. Thus, mutations in the virus that occur at the reverse transcriptase level will not affect photodynamic destruction [33].
Studies focusing on the possible development of viral resistance are extremely scarce and little is known about the recovery of viral viability after consecutive photodynamic treatments.
5.2. Bacteriophage Resistance and Viability Recovery after Photosensitization
Concerning bacteriophages, there is only one study focusing on the possible development of viral resistance after photosensitization [68]. After 10 consecutive cycles of photodynamic treatment, a T4-like phage, in the presence of the tricationic porphyrin 5-(pentafluorophenyl)-10,15,20-tris(1-methylpyridinium-4-yl)porphyrin (Tri-Py+-Me-PF) at 5.0 μM under white light irradiation, exhibited no changes in the rate of photoinactivation during the course of the experiments, meaning that no resistance was observed. If phage resistance would occur, important reductions on phage photoinactivation efficiency would be detected between experiments. Besides that, T4-like phage did not recover its viability after exposure to Tri-Py+-Me-PF during 120 min of irradiation [68]. In a preliminary study by Perdrau and Todd [12], all attempts at reactivating the inactivated Staphylococcus phage by MB were unsuccessful.
6. Factors Affecting Viral PDI
6.1. Effect of the Number of Charges, Symmetry, Size of Meso Substituent Groups and Photosensitizer Concentration
It has been shown that the location and binding site of the PS, which is highly dependent on the structure and intramolecular charge distribution, is an important factor in microbial PDI [143,147].
In terms of molecular structure, molecular charge is important in determining antimicrobial activity. Positively charged PS are generally more efficient and can act at lower concentrations than neutral and anionic PS molecules [144]. The positive charges on the PS molecule appear to promote a tight electrostatic interaction between the positively charged PS and the negatively charged sites at the viral capsids and envelopes, orientating the PS toward sites which are critical for the stability and metabolism of a particular microorganism [44,147,148]. This kind of association increases the efficiency of the photoinactivation process.
Cationic PS photodamage can be induced in nucleic acid or viral outer structures by PS binding or by PS localized in its vicinity [136]. For instance, it is more likely that positively charged PS will be effective in causing nucleic acid damage than will neutral or anionic congeners, which mainly act against the outer side of the microorganism [149].
The symmetry and the size of the chain of meso substituent groups also affect the photodynamic effect. PS with opposite charged groups are more symmetrical than PS with adjacent charged groups. The adjacent positive charges in the PS macrocycle should result in a molecular distortion due to electrostatic repulsion [150]. The toxicity of a PS can be modulated by the introduction of selected substituents on the macrocycle periphery. In this way, the physicochemical properties of a synthetic PS can be manipulated in order to enhance its interactions with the structural features of the viruses, such as viral capsids, and to minimize the interactions with plasma membranes or mammalian cell membranes [44].
The amphiphilic nature of a PS is another important feature affecting PDI efficiency and can be modulated by the introduction of adequate functionalities in the macrocycle periphery, such as different numbers of positive charges, an asymmetrical charge distribution, or introduction of aromatic hydrocarbon side chains [16,151].
PS concentration is also an important parameter that must be taken into account since viral PDI was shown to be strongly influenced by PS concentration. Increasing the PS concentration reduces the time needed to achieve complete viral inactivation, thus increasing the efficiency of a particular PDI protocol [66].
6.1.1. Mammalian Viruses PDI
Complete inactivation of VSV (4.2 log) can be obtained by treating it with 1.0 μM of the anionic phthalocyanine derivative AlPcS4 and 5 min illumination with red light. For the neutral phthalocyanine derivative (Pc4), complete inactivation (4 log) was achieved using a much lower amount of PS (4.5 nM) in combination with 10 min illumination [82]. The inactivation of VSV in PBS showed a linear relationship with illumination time [82]. Inactivation of the fusion activity of VSV, influenza and Sendai viruses was reached with nanomolar concentrations of hypericin and rose bengal and was absolutely dependent upon light and increased with increasing time of illumination [30]. HAV in PBS or plasma was completely inactivated within 10 min (>3.7 log) by the cationic symmetric porphyrin meso-tetrakis(1-methylpyridinium-4-yl)porphyrin. In contrast, inactivation of HAV to 3.6 log with the anionic symmetric porphyrin meso-tetrakis(4-sulfonatephenyl)porphyrin required 90 min [44]. The rate and extent of inactivation appeared to vary with the nature of the meso substituent groups [44]. HIV and VSV lost infectivity upon illumination with hypericin and rose bengal in a concentration-dependent manner [30].
6.1.2. Bacteriophage PDI
MS2 phage inactivation has been observed with neutral porphyrin derivatives. However, this required higher irradiation periods (30 min) than for the cationic ones (1 min) [44]. Neutral glycosylated substituted porphyrins can also significantly photoinactivate the T7 phage [64,87]. The T4-like phage PDI was achieved by exposing the phage in the presence of six cationic porphyrins at different concentrations (0.5, 1.0 and 5.0 μM) to white light for 270 min. The results showed that phage photoinactivation varied according with the PS concentration, with higher concentrations being the most efficient ones [66]. The T4-like phage PDI also varied with the number of porphyrin charges, with tri- and tetracationic porphyrin derivatives being more effective in viral inactivation that the dicationic ones, which inactivated the phage below the limit of detection. Tetra- and tricationic porphyrin derivatives (meso-tetrakis(1-methylpyridinium-4-yl)porphyrin and 5-(pentafluorophenyl)-10,15,20-tris(1-methylpyridinium-4-yl)porphyrin, respectively) lead to complete T4-like phage inactivation (~7 log) after 270 min of irradiation with 40 W m−2 [66]. This tetracationic porphyrin showed similar results in another study (7 log of reduction) for lambda phage inactivation, when irradiated with light of 658 nm [58]. Increasing porphyrin concentration at a fixed light dose leads to increased viral inactivation [58]. A concentration-dependent effect was also detected with a porphyrin derivative [87], but over 2.0 μM of PS the process was saturated. A further increase in porphyrin concentration did not lead to a higher inactivation rate of T7 phage. Aggregation and/or photobleaching of PS are likely explanations [87]. Cationic meso-tetrakis(1-alkylpyridinium-4-yl)porphyrin derivatives with different alkyl substituent groups were tested for MS2 phage inactivation but, with the exception of 5,10,15,20-tetrakis(4-sulfonatophenyl)porphyrin, showed toxicity even in the absence of light [44].
In a study conducted by Gábor et al. [64], the porphyrin derivative with symmetrical glycosylated groups was found to be twice as effective as the asymmetrical one on the inactivation process of T7 phage. According to Costa and colleagues [66], the rate of T4-like phage inactivation was also dependent on the lipophilic character of the meso-substituent groups. The presence of a lipophilic aryl group in one of the meso positions of the porphyrin core appears to have an important role in phage inactivation, affecting the rate and efficiency of T4-like phage [66]. Casteel et al. [44] have also observed differences in the photoinactivation rate of MS2 phage when they used PS with different alkyl substituent groups and concluded that the rate and extent of inactivation appeared to vary with the nature of the meso substituent groups.
6.2. Effect of Different Light Sources and Fluence Rate on Antimicrobial PDT
PDT requires a source of light to activate the PS by exposing it to visible or near-visible light at a specific wavelength [152]. The light source for PDT must also exhibit suitable spectral characteristics coinciding preferentially with the maximum absorption wavelength range of the PS, applied in order to generate enough ROS to produce an efficient toxic effect [153].
In parallel with the advances in chemistry (related with the discovery and synthesis of new and more efficient PS) there has also been much activity in developing new light sources, better suited for the photosensitization process. Briefly, these include user-friendly lasers frequently based on solid state laser diodes, as well as inexpensive light emitting diodes (LED) and filtered broad-band lamps [154].
PS activation has been achieved via a variety of light sources, such as arc plasma discharge lamps, metal halogen lamps, slide projector illumination assemblies, and a variety of lasers. For treatment of larger areas, non-coherent light sources, such as tungsten filament, quartz halogen, xenon arc, metal halide, and phosphor-coated sodium lamps, are in use. Recently, non-laser light sources, such as LED, have also been applied in PDT. These light sources are much less expensive and small, lightweight and highly flexible, its lifetime can reach up to one hundred thousands hours, and can be manufactured to wavelengths that activate commercially available PS [152,155,156,157,158,159].
At first glance, the available literature on fluence rate effects for PDT seems contradictory. Some studies indicate less damage at low fluence rate, others indicate more killing at lower, compared to higher, fluence rates for the same total fluence and some indicate no influence of fluence rate at all [152,157,158]. A reduction in the fluence rate lowers the rate of oxygen consumption, thereby extending the radius over which singlet oxygen may be formed and consequently increasing the phototoxic effect [159]. Qin et al. [160] showed that an increase in the fluence rate increases microbial damage, although, it seems to have an upper limit of photons to observe this effect. Since each PS molecule can only absorb one photon at a time, when the number of light photons bypasses the number of PS molecules, the PS will no longer be able to absorb the photons “in excess” and the rate of PDI will not increase. In fact, if the number of photons is higher than this limit, the antimicrobial effect will decrease because the dye in suspension will not absorb all the excess light [160]. Schindl et al. [161] referred that the biological effect of light depends on the fluence, irrespective of the time over which this dose is delivered. Maclean et al. [162] also indicate that the inactivating light may be applied at high irradiance over a short time or at lower irradiance over a longer time. A numerical model, assuming that the rate of photodynamic damage occurring at time t is proportional to the fluence rate at that time and the local concentrations of PS and oxygen can be established. However, according to this model, relatively low fluence rates can be nearly as effective as high fluence rate sources if applied over the same period of time [163].
There is also a direct correlation between the phototoxic effect and the PS concentration and light fluence. With a lowering of the PS concentration, more light has to be applied to achieve identical effects, and vice versa. Lower doses of PS require higher activating light fluences, and higher fluence requires a longer duration of light application [96].
6.2.1. Effect of Light on Mammalian Viruses PDI
The effects of dengue virus inactivation were increased with the increase of MB concentration, the enhancement of power density of the light source and the extension of illumination time, as well as the decrease of illumination distance. This enabled the narrow bandwidth light system to kill or inactivate the enveloped virus at much greater distance in much shorter time [74]. VSV in the presence of MB was rapidly inactivated by red (provided by LED incident light at 272 W cm−2) or green-yellow light (provided by low-pressure sodium lamps at a fluence rate of 165 W cm−2) but slower by white light (provided by a bank of fluorescent tubes at a fluence rate of 42 W cm−2) [46], showing that higher power densities produce a high rate of viral inactivation than low fluence rates. Wagner et al. [164] also showed that red light of 9 W m−2, given at a total dose of 1.8 × 104 and 3.2 × 104 J m-2, inactivated MB-treated VSV by 6 and ≥7 log, respectively. VSV inactivation was linearly dependent on the fluence rate of red light illumination [165].
6.2.2. Effect of Light on Bacteriophage PDI
In terms of what is known about phage PDI, only one study focusing on the effect of different light sources and power densities [67] exists. In this study, cationic porphyrin derivatives (meso-tetrakis(1-methylpyridinium-4-yl)porphyrin and 5-(pentafluorophenyl)-10,15,20-tris(1-methylpyridinium-4-yl)porphyrin), when irradiated with different sources of light (fluorescent PAR lamps, sun light and halogen lamp) with fluence rates ranging from 40 W m−2 to 1690 W m−2, efficiently photoinactivated non-enveloped phages. All light sources tested lead to reductions of about 7 log for the somatic T4-like phage. However, the rate and the extent of inactivation were dependent on the light source, namely when low fluence rates were used (40 W m−2) and on the energy dose, being considerably more effective when light was delivered at a lower fluence rate. However, depending on the light source used, different irradiation periods were required to inactivate T4-like phage to the limits of detection. The results also showed that the efficacy of T4-like phage inactivation, using the same fluence rate, was dependent on the light source used, in particular when the light is delivered at a low fluence rate. M13 phage was phototreated with 5.0 μM MB and was inactivated in an irradiation dose-dependent manner [52]. Kastury and Platz [58] showed that increasing the concentration of a PS at a fixed light dose leads to increased viral inactivation as does an increase in the total light exposure at a fixed PS concentration. The inactivation rate of T1 bacteriophage increased with increasing fluence rate, indicating that the distance of the sample from the light source is a variable which must be controlled [73]. At higher PS concentrations, the inactivation rate reaches a maximum and then decreases, because the filtering effect of the dye decreases the effective fluence rate [73]. In a simple model purposed by Lee et al. [56], the phage survival ratio can also be considered as a decreasing exponential fraction of the light fluence (assuming that the fluence is uniform throughout the system).
7. Conclusion
The efficiency of different types of PS in viral PDI has been proved for different types of mammalian viruses and bacteriophages, whether they are enveloped or non-enveloped, for either DNA or RNA viruses. Even though enveloped viruses are more easily inactivated than non-enveloped ones, several studies confirm that non-enveloped mammalian viruses and phages can be efficiently inactivated by PDI. The type of viral nucleic acid has not been described as an important factor affecting viral photoinactivation but, as far as it is known, no studies specifically focus on the photoinactivation behaviour of DNA and RNA viruses. However, RNA phage MS2 was highly susceptible to photoinactivation when compared with DNA phages under the same conditions of photosensitization.
The type of mechanisms involved in the process of viral photosensitization was already elucidated and singlet oxygen and free radical species were identified as important contributors for an effective viral PDI. However, the contribution of singlet oxygen seems to be more pronounced in mammalian viruses and bacteriophage PDI. There are, however, few studies simultaneously comparing the contribution of both types of mechanisms (type I and type II) involved in viral PDI. The primary targets for the photoinactivation of viruses, whether treating mammalian viruses or phages, are the outer structures. Although there are several studies about the specific effects of PDI on viral proteins, for different types of mammalian viruses and phages, there are no studies concerning the specific effects of PDI on viral lipids. However, it has been clearly shown that enveloped viruses are more easily inactivated than their non-enveloped counterparts, which imply that the lipids present on viral envelopes are important targets of viral PDI.
PS are effective in inactivating the phages to the limits of detection in a way that they do not recover viability, avoiding the development of viral resistance. Nothing is known yet for the particular case of mammalian viruses but, as the viral targets are the same for mammalian viruses and phages, it is also expected that no resistance will be developed in the case of mammalian viruses. Besides that, antiviral PDI is equally effective whether the mammalian virus is sensitive or resistant to conventional antiviral agents. Taking into account all these advantages, PDI for viral inactivation can be regarded as a promising alternative therapy to conventional antiviral treatments, namely for the disinfection of blood and blood products, preventing viral contamination and for the treatment of wound and burn infections. Viral PDI has a fast mode of action and has also the additional benefits of being more economical and an environmental friendly technology, which might be successfully used also in the environmental field for wastewater, drinking water and fish-farming water disinfection.
Different PS concentrations and different light sources and fluence rates were tested, showing that they are important PDI parameters that must inevitably be taken into account when a viral photosensitization protocol has to be elaborated. The inactivation of mammalian viruses and phages can be attained at micromolar-level PS concentrations and different light sources are equally effective, depending on the final dose at which the viruses are exposed to. Besides that, PS can also be modulated by the addition of different meso substituent groups and positive charges in order to facilitate their interactions with the viruses, making them more efficient for mammalian viruses and phage PDI.
The similarity of the results obtained for mammalian viruses and bacteriophages show that they exhibit a similar behaviour when submitted to viral photoinactivation techniques: (i) the PS used for viral PDI were equally effective in the photoinactivation of mammalian viruses and bacteriophages; (ii) the mechanism of mammalian viruses and bacteriophage photosensitization involves the production of singlet oxygen (type II mechanism) with a slight contribution of free radical species (type I mechanism); (iii) singlet oxygen and free radicals were shown to affect viral nucleic acids and also the proteins and lipids present in the mammalian viruses and bacteriophage outer surfaces, with the latter being considerably more affected by PDI; and (iv) the rate and extent of mammalian viruses and phage PDI is also affected by the same factors, like the PS concentration and number of positive charges, the nature and position of meso substituent groups, the fluence rate and energy dose. Consequently, it is important to persist in the development of more PDI phage studies to clarify some aspects of viral PDI, such as influence of viral nucleic acid type (DNA or RNA) in the photoinactivation efficiency and the possibility of viral resistance development and viability recovery after photosensitization. It will also be important to study the synergistic effect between viral PDI and antiviral classical methodologies using bacteriophages as models of mammalian virus’ photoinactivation.
Viruses. 2012 Jul; 4(7): 1034–1074.
Published online 2012 Jun 26. doi: [10.3390/v4071034]
PMCID: PMC3407894
PMID: 22852040
Liliana Costa,1 Maria Amparo F. Faustino,2 Maria Graça P. M. S. Neves,2 Ângela Cunha,1 and Adelaide Almeida1,*
Author information Article notes Copyright and License information Disclaimer
This article has been cited by other articles in PMC.
Go to:
Photodynamic Inactivation of Mammalian Viruses and Bacteriophages https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3407894/
.png)
.png)
.png)
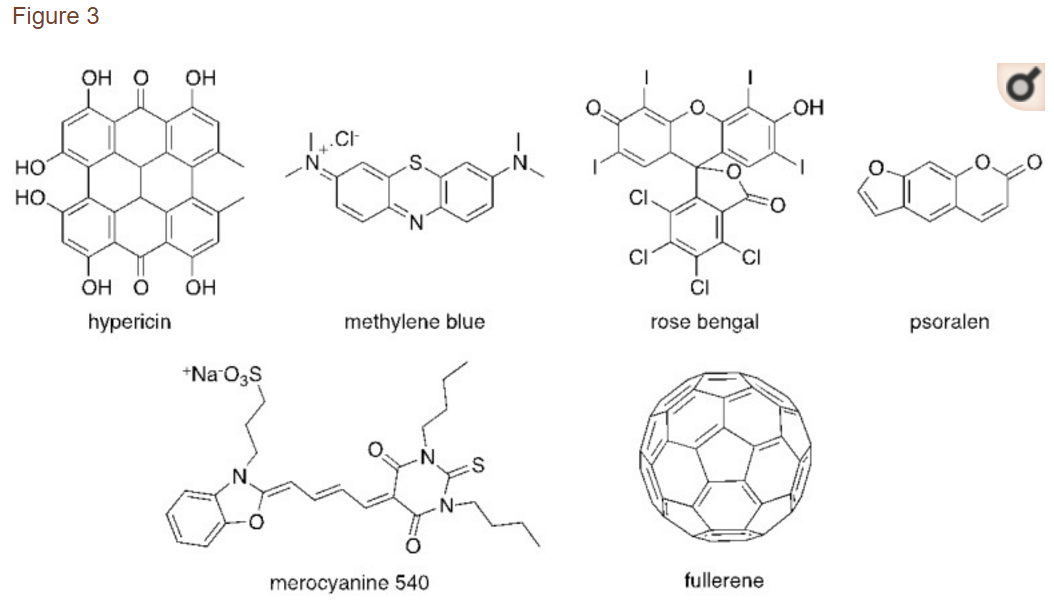
.png)
.png)